Left sided lobectomies
Introduction
Lobectomies can be performed in case of both malignant and non-malignant lung diseases. Preoperative assessment is fundamental and should always include lung function tests, as the patients should have an adequate pulmonary reserve in order to tolerate the resection. Echocardiography and spiroergometry should be performed, if needed, in correlation with the patient’s previous medical history.
In patients with proven or suspect of lung cancer, further preoperative staging is required and it includes chest computed tomography, positron emission tomography scan, and, if indicated, endobronchial ultrasound-guided mediastinal lymph node biopsy or mediastinoscopy. In planning the resection, the surgeon should be sure that the tumour can be dissected from the main central structures, so that a safe, and oncological radical, resection can be performed.
Video-assisted thoracoscopic (VATS) lobectomies are increasingly performed as standard for early stage lung cancer, so the indication for open approaches are evolving and often depends on the surgeon’s experience with VATS techniques (1,2).
Open lobectomies are still generally performed in case of large tumours or in more difficult resections, as the open technique allows more space and so a better access and control of the intrathoracic structures.
Operative technique
Positioning
As in most thoracic surgery procedures, whether done as a VATS or open, the procedure is performed in right lateral decubitus position (Figure 1).
The most important but also avoidable hazards regarding the positioning are injuries due to nerve stretching or compression at pressure points. Every clinic should have their own positioning standard. An axillary roll should be placed to prevent the injury of the brachial plexus or shoulder. All pressure points should be padded. The patient is held and stabilized by a stiff bean bag, which should hug the patient bilaterally for better support.
Incision
The standard approaches be performed through a posterolateral thoracotomy or a muscle-sparing anterolateral thoracotomy, as preferred from the authors (Figure 1).
The Dartevelle incision gives a better access to the apex, whereas the hemiclamshell incision can be used to have a better access of the anterior mediastinum and subclavian vessels.
Exposition
After the beginning of one-lung ventilation the skin incision is performed on the forth intercostal space, in case of anterolateral thoracotomy. Whenever possible the latissimus dorsi muscle should only be elevated and retracted, but not incised. The serratus anterior is also only split in the direction of his fibres. The underlying intercostal muscles are cut carefully in the lower portion of the intercostal space to avoid injuries of the neurovascular bundle. The intercostal incision is performed with the help of a Mikulicz liver retractor posteriorly to the level of the paraspinal muscles and anterior to the curve of the ribs. Then one (or two) rib spreaders can be positioned, according to the surgeon’s preference.
Next step is the mobilization of the lung to get a better exposition to verify the indication or exclude possible contraindications, e.g. tumorous pleural dissemination or invasion of hilar structures.
Left upper lobectomy
Operation
Depending on the surgical indication, the different steps of the surgery can be performed in a different order. As lung cancer is the indication for most lobectomies, we will describe the classical technique for left upper lobe resections, starting with the division of the superior pulmonary vein (SPV).
- Isolation and division of the SPV:
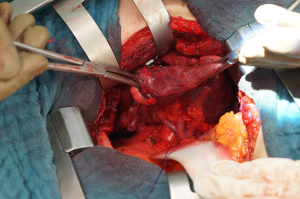
The left superior pulmonary vein is to be exposed by retracting the lung inferiorly and incising the anterior mediastinal pleura, paying attention to the left phrenic nerve. After opening the pleura, the superior and inferior pulmonary vein should be identified to exclude a common confluence of the pulmonary venous blood support (Figure 2). Particular attention should be payed to the lingular branches, making sure that they originate from the SPV. Now the vein can be cleared with a blunt preparation from all overlying tissue in its whole circumference. The vein can be grasped with broad forceps/graspers during the dissection. Note that behind the superior margin of the SPV normally is located the arterial branch for the 3rd segment (A3). Then the SPV can be divided with a stapler or a suture ligature. In this last case, a vascular clamp is applied proximally on the SPV, then the vein is divided and closed with a vascular suture, to avoid the ligature falling off the SPV. Sometimes the extrapericardial length of the vein is too short to put a ligature safely, so in this particular situation the pericardium has to be opened and the vein has to be divided intrapericardially. - Dissection and division of the arterial branches for the upper lobe:
Continuing the opening of the mediastinal pleura overlying the aortopulmonary window and then posteriorly along the anterior margin of the esophagus allows the exposition of the underlying pulmonary artery (PA).
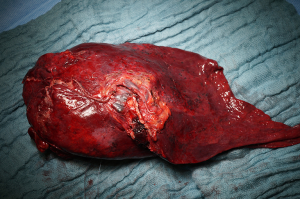
The dissection of the segmental arteries can be very challenging because of their numerous anatomic variations. The most common order should be: A3, A1, A2, A4 and A5 (or A4+5). Essentially, every surgeon has to identify every branch of the PA based on its position and topography. To start, retract the lobe inferiorly and the first branches that should be identified are the A3 and right behind the A1+2a. Be aware that there can also be a common branch for A3+1+2, which has to be dissected very carefully. Then, retracting the lung anteriorly, occasionally a small branch can be identified as the A1+2b+c. The fissure should be completely opened or at least long enough to identify the last posterior branches to the left upper lobe (A4+5) and the first lower lobe branch (A6). Normally the A4+5 is to be found distally from A6, but it can also come from A8. All the branches are to be separately ligated, stapled or clipped (Figure 3). Finally, complete the anterior and posterior fissures if not preformed yet. - Dissection and division if the left upper lobe bronchus:
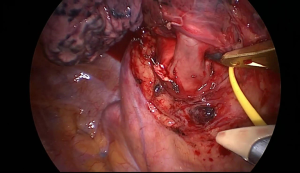
Next and last step is to dissect and divide the left upper lobe bronchus. Retracting the lobe anteriorly, roll the interlobar pulmonary artery posteriorly and try to palpate the gap between the two lobar bonchi. After identifying the upper lobe bronchus, the best way to dissect it is to bluntly strip with a peanut the bronchus and the peribronchial lymph nodes distally to become part of the lobar specimen. Usually, the best way to divide the bronchus is using a stapler. In positioning the stapler, make sure that the whole bronchus is included but that the lower lobe bronchus is not constricted. To check this, the anaesthesiologist can help by inflate the lower lobe or pass the bronchoscope in the left bronchial system (Figure 4).
Completion
In case of lung tumors, a mediastinal lymph node dissection and should always be performed for staging. Lymph nodes in station 5 and 6 are removed from the space between the aorta and the main PA. Station 4 lymph nodes can be reached by retracting the left PA and exposing the tracheobronchial angle anteriorly.
The dissection in the aortopulmonary window should be carried on close to the PA to avoid the injury of the recurrent laryngeal nerve.
The infra-carinal lymph nodes (station 7) should be approached from behind, opening the plane between the esophagus and left main bronchus.
The inferior pulmonary ligament should also be taken down, if not already done. In doing this, the lymph nodes in station 8 and 9 can be removed and the lung can be mobilized, allowing a better expansion of the remaining lung in the thoracic cavity.
Note that in case of pulmonary sequestration a systemic artery could be located in the pulmonary ligament, so the dissection must be particularly careful.
Station 10 and 11 lymph nodes are normally removed during the procedure together with the lobe or singularly during the exposition of other structures (3).
Always check for air leaks of the remaining lower lobe and the bronchial stump under warm saline irrigation with a pressure up to 30 cm H2O. A careful hemostasis should be performed prior closure of the chest. One or two chest tubes are then placed and the ribs are approximated with four single heavy absorbable pericostal sutures. Before rib approximation, the remaining lung should be inflated, checking that it does not rotate during this manoeuvre.
Left lower lobectomy
Operation
The left lower lobectomy is under normal conditions considered the easiest anatomic resection in lung surgery and the surgery involves three main steps, similarly to the left upper lobectomy.
- Dissection and division of the inferior pulmonary vein (IPV)
The first step is to carefully divide the pulmonary ligament using the cautery until finding station 9 lymph nodes and the inferior margin of the IPV. Then the anterior and posterior mediastinal pleura is opened to get full access to the IPV. The IVP is then dissected bluntly, circled and then divided with a stapler order manually with ligatures. As for the left upper lobectomy, it is always mandatory to check the presence of the SPV before dividing the IPV. In case of large tumors invading the IVP, it may be necessary to open the pericardium and divide the vein intrapericardially. - Dissection and division of the interlobar pulmonary artery (ILPA)
In case of complete fissure the ILPA can be clearly identified in the interlobar fissure. If the fissure is incomplete, the dissection of the fissure should be performed with cautery, peanuts or scissors until finding the station 11 lymph nodes: posteriorly to these nodes normally is located the ILPA. The small bleeding caused from the fissure dissection can be stilled with cauterization or using metallic clips.
Normally the anatomy of ILPA consists in a single superior segmental branch and in the common basal segmental branch. After identification and dissection, the two branches can be divided separately using a stapler or ligatures.
Rarely, arterial branches for the upper lobe originates from the ILPA, so during the dissection the surgeon must recognize this anatomical variation and preserve the upper lobe branch. - Dissection and division of the lower lobe bronchus
The lower lobe bronchus is normally located behind the ILPA, so after the division of the ILPA it is relatively easy to find it. After its identification, the peribronchial tissue is swept toward the specimen and then the bronchus is divided using a stapler, after checking on the upper bronchus inflating the lung or by bronchoscopy.
Completion
The rest of the steps are the same as for the left upper lobectomy: mediastinal lymphadenectomy, hemostasis and proper closure of the chest after tube placement.
Comments
- Use of the stapler
- Good dissection of the planes to have enough space to insert the anvil;
- It may be helpful to pass the stapler through a separate incision, in case of difficult angles. This incision can then be used for the chest tube;
- Always encircle the vessels with a vessel loop/ligature, as it can be useful to retract and to expose better the entry point of the stapler;
- The stapler should not be forced, but should pass without resistance;
- Be careful of the structures located in the area around the target, as they can be pushed away from the anvils;
- Have a sponge stick ready when working on the vessels.
- PA branches of smaller size can be clipped with polymer ligating clips, like it is usually done in the minimally invasive technique.
- The introduction of an enhanced recovery pathway for thoracic surgery patients should improve the quality of care, accelerating the recovery and shortening hospital stay postoperatively. It means that the management of patients should be structured starting from the preoperative preparation and it should involve all medical figures involved in patient care and deal with all its aspects (4).
- Controlling the main pulmonary artery during difficult dissection allows a better vascular control in case of arterial bleeding.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. Ann Thorac Surg 2008;85:1857-65. [Crossref] [PubMed]
- Zhang W, Wei Y, Jiang H, et al. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290. [Crossref] [PubMed]
- Scarci M, Solli P, Bedetti B. Enhanced recovery pathway for thoracic surgery in the UK. J Thorac Dis 2016;8:S78-83. [PubMed]
Cite this article as: Bedetti B, Schnorr P, Patrini D, Scarci M, Schmidt J. Left sided lobectomies. Shanghai Chest 2017;1:6.