Tracheal sleeve pneumonectomy: indications and surgical operative techniques
Introduction
Tracheal sleeve pneumonectomy is a challenging surgical procedure for resection of cancers involving lung, lower trachea, carina and tracheobronchial angle. The most common indications are non-small cell lung cancers, bronchogenic carcinoma, carcinoid tumor, adenoid cystic carcinoma or inflammatory strictures. Other common indication is a positive resection margin after a standard pneumonectomy.
This type of surgery is currently still infrequent, because of the complexity and the high risk of complications. The recent improvement of surgical techniques, different ventilation modalities and postoperative management has reduced dramatically the mortality and morbidity, especially in high volume centers (1).
The careful selection of the patient is the central point for a successful operation (Table 1). Absolute contraindications include insufficient pulmonary reserve, impaired cardiac function, N2/N3 nodal disease, and excessive airway involvement (2).
Table 1
| Type of evaluation | Tests required |
|---|---|
| Physical examination | BMI, general condition |
| Hematochemical and urine analysis | Standard preoperative ematochemical analysis |
| Pulmonary function | Basal and stress spirometry (PFR) |
| Diffusing capacity of the lung for carbon-monoxide (DLCO) | |
| Arterial blood gas analysis | |
| Cardiac function | Cardiologic evaluation |
| Electrocardiography | |
| Transthoracic echocardiography | |
| Cardiopulmonary exercise testing | |
| Imaging | Chest X-ray |
| Chest, upper abdomen computed tomography (CT) with i.v. contrast | |
| Brain MRI/brain CT with i.v. contrast | |
| Positron emission tomography (PET) | |
| Perfusion scan | |
| Bronchoscopy with biopsies to evaluate the airway involvement | – |
| Assessment of mediastinal lymph nodes | Mediastinoscopy can exclude N2/N3 disease and mobilize pretracheal plane for an additional mobility |
| Endobronchial ultrasound with transbronchial fine needle aspiration cytology is an alternative to mediastinoscopy especially in the case of previous intervention on the anterior mediastinum |
i.v., intravenous.
Relative contraindications include prior neoadjuvant radiation (>45 Gy) and steroid use, because of higher risk of anastomotic dehiscence.
Tips and tricks
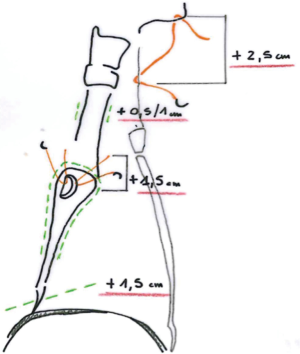
- Pre-operative evaluation by bronchoscopy and CT scan: a tension free anastomosis is possible only if the tumor does not extend beyond 1.5 cm of the main bronchus or 2.5–3.5 cm of the distal trachea. The total distance of the airway resection must be less than 4 cm because of the risk of anastomotic dehiscence.
- Steroids should be withdrawn at least 3–4 weeks before surgery.
- Mediastinoscopy should be performed at the same time of planned surgery to prevent mediastinal scarring that could reduce tracheal mobility (3).
- Is not recommended to repeat mediastinoscopy also in order to avoid excessive devascularization.
- EBUS is a reliable node-staging technique for its high sensibility and specificity, and can avoid the problem of scare or devascularization when mediastinal staging is planned long before surgery
Anesthesiological approach
Preparation
In this scenario a cooperation between surgeons and anesthesiologists is fundamental (4).
There are several different types of ventilation that can be chosen:
- Long, armored, flexible single-lumen endotracheal tube: typically placed in the trachea at the beginning of surgery. It can be taken forward the left or right mainstem bronchus during the procedure. Once the airway has been transected, the patient is ventilated with a cross-fields sterile endotracheal tube in the mainstem bronchus, passed by the anesthetist and connected with a sterile tubing system.
- During the surgical anastomosis the tube can be intermittently slided out. After the completion of the posterior wall of the anastomosis, the cross-field tube can be removed and the original endotracheal tube can be inserted in the mainstem bronchus.
- Double-lumen tube to allow single lung ventilation: this type of tube is rigid and difficult to pass through distal carina.
- High frequency jet ventilation: a technique of mechanical ventilation that uses very high rates (>100 breaths/minute) with small tidal volumes (100–150/minute) delivered by a small catheter into the mainstem bronchus through the retracted single or double-lumen endotracheal tube. This technique guarantees ventilation without the side effects of positive pressure, but can lead to carbon dioxide retention. Because of the small diameter of the catheter, surgical reconstruction is much easier (4).
- Intermittent apneic ventilation: when the oxygen saturation decreases, the endotracheal tube is inserted across the open airway and the patient is hyperventilated with 100% oxygen. This results in a rise of oxygen saturation so that surgeons can go on preparing surgical anastomosis after having thrown back the tube.
- Long and thin single-lumen tube of 45 cm with a self-expanding cuff.
- Cardiopulmonary bypass/ECMO: in our experience it’s better to plan tracheal sleeve pneumonectomy without these supports. The use of extracorporeal oxygenation is restricted to situations in which gas exchange is not sufficient during single lung ventilation or in case of major bleeding during surgery.
Surgical approach
Median sternotomy, anterolateral thoracotomy, posterolateral thoracotomy, bilateral thoracotomy, hemi-clamshell or clamshell can be used to approach lower trachea, carina and tracheobronchial angle.
The type of incision varies according to the site of the tumor, and therefore patient’s positioning depends on this choice.
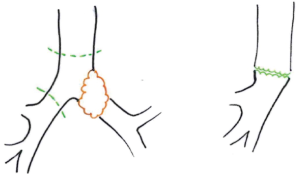
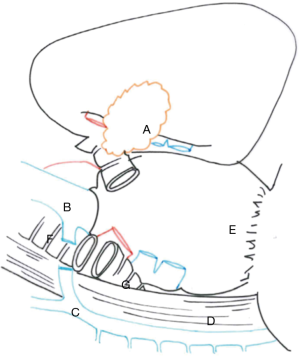
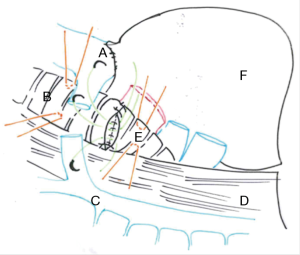
In this chapter we won’t talk about carinal reconstruction without pulmonary resection, so we have just illustrated the main reconstructions types below (Figure 1) (5).
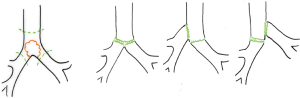
Right carinal sleeve pneumonectomy
Right sleeve pneumonectomy is the most frequent pulmonary resection with carinal reconstruction. Several surgical approaches are possible and the choice depends on the surgeon’s preference as well as on the local pathological condition (Figures 2,3).


Transthoracic approach
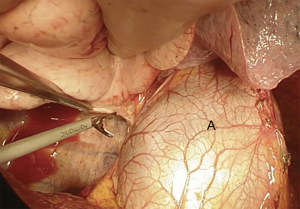
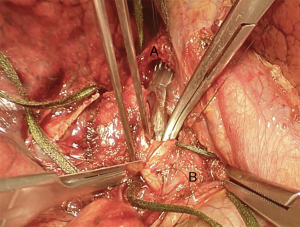
We suggest a standard right posterolateral thoracotomy in fourth intercostal space. First of all we divide the inferior pulmonary ligament to free the whole lung (Figure 4). The azygos vein can be divided to improve exposure of the carinal space (Figures 5,6). We prepare the pretracheal plane, open the pericardium, suspend the trachea as well as the mainstem bronchus at both sides (Figures 7,8). We suggest to use intraoperative bronchoscopy to identify the proximal and distal extend of the tumor and the point where transect the airway. For ventilation we prefer to use the first described method, so a cross-field sterile endotracheal tube is positioned (Figure 9). Endotracheal tube is withdrawn and the trachea is transected. Mid-lateral stay sutures 2-0 are placed on its wall (Figure 10). After that the left mainstem bronchus is cutted and inspected (Figure 11). The right pulmonary artery and veins are encircled and ligated.
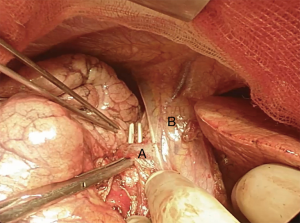
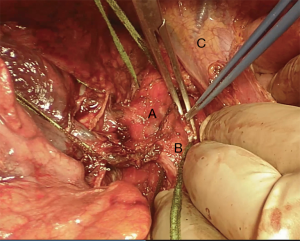
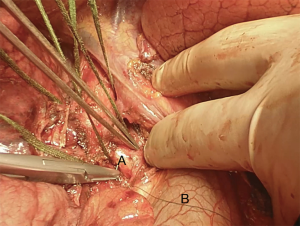
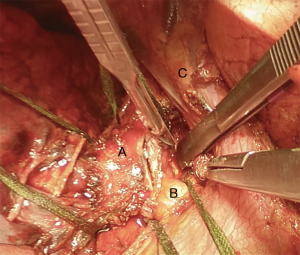
Subsequently we proceed with en bloc removal of the specimen and start with reconstruction. Bronchial margins must be examined, so frozen sections are imperative: in case of positive margins, a balance must be struck between the need of R0 resection and concern about reconstructing the airway.
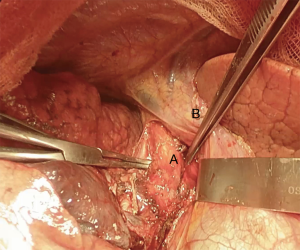
Bronchial traction absorbable sutures with 2-0 or 3-0 are placed on tracheal wall to stabilize the structure and give the surgeon the feeling of tension of the suture (Figure 12) (2).
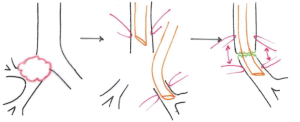
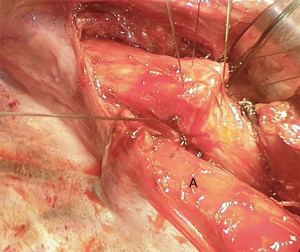
There are several ways to perform the anastomosis: we prefer to use 4-0 absorbable interrupted sutures with knots tied outside (Figure 13), beginning with back row and ending with front row, starting to tie the knots gradually in a symmetric sequence from the opposite sites of the suture. Any size disparity between distal trachea and left mainstem bronchus is corrected by stretching the bronchial lumen to the size of the tracheal one (6).

Before closing the sutures, the patient’s neck is flexed (Figure 14) to move closer the distal trachea to the relatively fixed left main bronchus. In fact, that applies to any anastomosis, is crucial the absence of traction for tissue healing. The anastomosis is inspected with flexible bronchoscope, and a pedicle flap of pleura or pericardial fat is passed around to separate the suture line from vascular structures (2).
Transternal approach
The patient is positioned supine with the neck extended and both arms abducted. The median sternotomy is performed in the standard way, but the incision can be extended cranially for a better control of the left brachiocephalic vein (7). After initial dissection, the left brachiocephalic vein and artery are isolated and surrounded. The anterior pericardium is opened. Ascending aorta is isolated, surrounded and retracted to the left, while the superior vena cava is retracted to the right, to expose the trachea. A careful dissection of the airway is performed primarily on the anterior planes to avoid injury to blood supply. After that we reach the right pulmonary artery on the side, dissect it and isolate it from the mainstem bronchus (8).
Transection of arterial and venous structures is performed. The posterior pericardium is incised to a wide exposure of the airway. The distal trachea and the proximal bronchi are dissected circumferentially and suspended. To increase the mobility of the trachea we suggest to carry out an extensive nodal dissection in this moment.
We recommend to perform intraoperative bronchoscopy to identify the extension of the tumor and decide where to transect the airway. The left mainstem bronchus is transected and inspected. Mid-lateral stay sutures 2-0 are placed to stabilize the structure and a cross-field sterile endotracheal tube is positioned for left lung ventilation. Endotracheal tube is withdrawn and the trachea is transected and a right pneumonectomy is performed.
Mid-lateral stay sutures 2-0 are placed on its wall to stabilize the structure. The sutures (interrupted absorbable 4-0), are placed usually from the midline of the membranous wall posteriorly carrying on to the mid-lateral stay suture (Figures 15,16) (2).
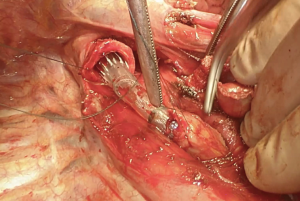
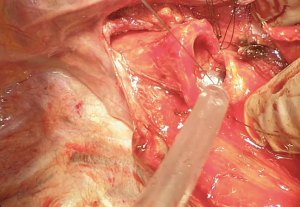
Flexion of the neck leads to approximation of the two sections of transected airway. Because a tension-free anastomosis is the key point, if tissue traction seems excessive it is necessary a further dissection of the trachea or the left mainstem bronchus. Eventually a left hilar release could be done, otherwise this procedure is normally performed via a left anterior thoracotomy (with an incision of the pericardium below the inferior pulmonary veins or circumferentially) (8).
The stitches are then tied in the same way that we described before. When the anastomosis is completed the original endotracheal tube is pushed in over the sutures for a standard ventilation.
Clamshell approach
The access to thoracic organs with the clamshell incision is similar to that obtained with the median sternotomy, but the clamshell incision allows larger bilateral exposure to the two pleural cavities and the pulmonary hilum, with sufficient exposure of the posterior surface of the airway. This approach is advantageous to achieve the maximum left hilar release when the airway reconstruction faces an extensive gap.
Sequential posterolateral thoracotomies or right thoracotomy associated with left video assisted thoracic surgery (VATS) are alternatives to clamshell incision.
Here a brief summary of a clinical case that you can see in the figures (Figures 4,6,7,8,10,11,13,15,16 17,18 19,20 ,21). This is a case of a 41-year-old female patient, affected by adenoid cystic tumor of the right main bronchus, which involves the carina and the first centimeter of the left main bronchus. Due to the necessity of resect a wide part of trachea, the best approach for this right sleeve pneumonectomy is a clamshell incision. This allows a major left hilar release with the maximum mobilization of the left main bronchus.
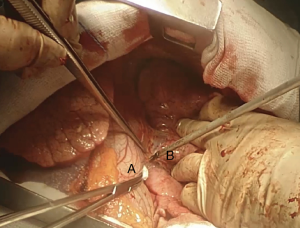
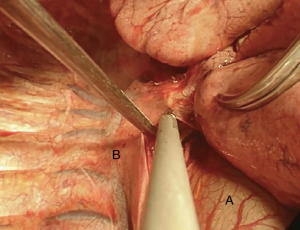

VATS approach
Recently, VATS approach to right sleeve pneumonectomy has been described. These little case series are restricted to single author’s experience, due to the technical challenging. Very short carinal resection seem to be the main indication for this approach (9,10).
Left sleeve pneumonectomy
This kind of tracheal resection (Figure 22) is much less frequent than right sleeve pneumonectomy due to the length of the left main bronchus and to the fact that tumors of this side very often invade the structures of the subaortic space.
Furthermore, this kind of bronchoplasty is more challenging because of the aortic arch, which covers the lower trachea and the right mainstem bronchus (6).
Surgery can be performed via different approaches. In every case it’s important to avoid devascularization of trachea and main bronchus, and to minimize airway tension by extended releasing maneuvers.
Thoracotomy approach
When the tumor invades the left mainstem bronchus with a limited involvement of the carina, a left thoracotomy is the best choice. It offers an excellent exposure of the pleural space and a sufficient access to the posterior mediastinum (6).
A left posterolateral thoracotomy is performed in fourth intercostal space. The aorta is suspended and retracted laterally and cranially to expose the aortopulmonary window (Figure 23). Pulmonary artery and aorta are separated. The anterolateral plane of the trachea and the right mainstem bronchus are dissected.
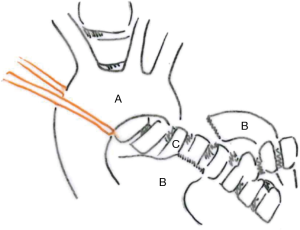
An extended lymph node dissection is performed. The hilar arterial and venous vessels are divided.
The trachea, the left and right mainstem bronchus are encircled.
The ventilation of the right lung with a cross-fields sterile endotracheal tube follows the cutting of the airway. Left pneumonectomy is performed.
Lateral traction absorbable suture with 2-0 are placed in the distal trachea and right mainstem bronchus. The anastomosis is achieved with a circumferential 4-0 interrupted suture with the knots tied in the outside, which starts at the farthest point from the surgeon. All stiches are sequentially placed before knots are tied.
Any size discrepancy between trachea and right bronchus should be adjusted with each stitch instead of tailoring the section margin (2). The patient’s chin is flexed to approximate the suture. First of all, it’s better to tie the traction stitches, and after the stitches on the cartilage side. The membranous sutures are the last one to be tightened.
Sternotomic approach
The median sternotomy allows a bilateral access to mediastinum with a good anterior exposure of the trachea (despite the presence of aortic arch that doesn’t allow the access to this part of the mediastinum). On the other hands the control of the left pulmonary veins is more difficult, and the access to the posterior part of mediastinum is limited and the presence of aortic arch does not allow to expose the entire surface of the airway.
After median sternotomy, the anterior pericardium is opened. The superior vena cava is encircled and retracted to the right, the aorta is retracted to the left leading to the exposure of the posterior pericardium.
For a better exposure of the left mainstem bronchus is necessary to retract the aorta to the right: this maneuver allows isolation and section of the right pulmonary artery follows by the two pulmonary veins (Figure 23) (5).
The endotracheal tube is retracted and the airway is divided with two sections: on distal trachea and on proximal right bronchus. Left pneumonectomy is therefore performed. The right lung ventilation depends on a cross-fields sterile endotracheal tube. We suggest to increase the mobility of the pretracheal plane by hilar release and by dissection of the tissue that cover the right bronchus and trachea. As usual, lateral traction sutures are placed on either side.
Unlike thoracotomic approach, in this situation the interrupted anastomotic stitches are placed in the membranous trachea first and tied inside. Afterwards interrupted 4-0 suture are placed in the cartilaginous trachea and tied outside.
Clamshell approach
The clamshell approach has the same advantages previously exposed for the right sleeve pneumonectomy; in addition, the combination of anterior view of the mediastinum and the lateral view of the left pleural cavity makes this approach particularly attractive for the left sleeve pneumonectomy.
Combined approach
A smart combined approach to left pneumonectomy with carinal resection includes the right thoracotomy for carinal resection and anastomosis between the trachea and the right main bronchus followed by a left VATS pneumonectomy (9,10).
Common auxiliary maneuvers and postoperative care
Anastomotic prophylactic coverage is advisable especially to isolate the suture line from the vascular structures immediately anterior to the anastomosis: pericardial fat, pleural flap, azygos vein flap or intercostal muscle flap are commonly used. We test air leakage by soaking anastomosis in sterile warm saline solution. Systematic lymph node dissection is a routine practice.
When a considerable tract of the trachea was resected, a heavy stich between the chin and the skin overlying the sternal manubrium is recommended to limit patient’s neck extension and to protect the airway anastomosis in the early postoperative day (~7 days) (2).
Postoperative care begins with a flexible bronchoscopy after the surgery to control the anastomosis and to clean up the secretions. Most of the patients are extubated in the operating room. The management is focused on systematic pulmonary toilet maneuvers with early mobilization, serial bronchoscopies and physiotherapic exercises.
To obtain the best pain control we suggest to use epidural anesthesia.
Perioperative wide spectrum antibiotic therapy is usually recommended and its duration is determined by the clinical status and the quality of the secretions. Caution intravenous fluids administration is required to avoid fluid overload.
Some authors suggest to use beta-blockers or calcium channel blockers to prevent atrial arrhythmias.
A flexible surveillance bronchoscopy is appropriate to evaluate the anastomosis before discharge from the hospital (~7 days postoperatively).
Tips and tricks
- Transternal approach allows a bilateral control of the airway and it is a good choice for tumor of the distal trachea only with minimal involvement of the mainstem bronchi (<2 cm).
- Lateral dissection to the proposed lines of transection should be limited to avoid devascularization of the airway (<2 cm). Same attention must be taken during lymph node dissection.
- To avoid injury of the left recurrent laryngeal nerve during the dissection we suggest to stay very close to the trachea.
- To reduce the tension of the suture a good maneuver is to dissect the anterior pretracheal plane (commonly during the mediastinoscopy) and release the hilum (Figures 4,8,18,19
). - Intraoperative bronchoscopy is very helpful to determinate the extension of the tumor. Use transillumination or a transbronchial needle to mark the exact point.
- Transection of the trachea and mainstem bronchus must be performed perpendicular to the axis.
- Perform a complete circumferential section of the airway without leaving rim of cartilage between the structures
- The distance of the resection line to the tumor should be >1 cm.
- Do not tailor trachea or bronchus to correct size discrepancy.
- Traction sutures should not be placed in the membranous wall, since they may tear as they are tied.
- Many authors prefer the technique in which running sutures are used for membranous part and interrupted sutures are used for the cartilaginous part.
- Intraoperative frozen-section examination of the proximal and distal resection line is mandatory.
- Wrap the anastomosis with intercostal muscle flap, pericardium or pericardial fat.
- Immediate post-anastomosis bronchoscopy is useful to check the suture.
- In few selected cases a temporary tracheotomy can be performed in the first days to facilitate direct aspiration of secretions.
Comments
Complications
The main cause of death is related to the development of respiratory failure:
- Adult respiratory distress syndrome (ARDS) and post pneumonectomy pulmonary edema: this syndrome occurs in the first 72 hours in 10% of patients with an associated mortality risk of 90%. The cause remains unclear but maybe it results from a combination of systemic inflammatory response, barotrauma, fluid overload and lymphatic interruption. Current recommendations include minimizing pulmonary artery pressures, minimizing mean and peak airway pressure, diuretics, non-invasive ventilation and fluid restriction.
- Anastomotic dehiscence: it occurs from 11% to 17% and it is mostly related to tension, faulty anastomotic technique or intraoperative devascularization with subsequent ischemia. The overall death rate is close to 44%. If there is any evidence of immediate anastomotic leak it is necessary a surgical repair. If dehiscence occurs, it’s necessary to protect airway with endotracheal ventilation, set up a multi-spectrum antibiotic therapy and drain the pneumonectomy space with a chest tube or performing an open thoracostomy. Necrosis and ischemia can be treated with hyperbaric oxygen therapy. Delayed stenosis and granulation tissue are considered late complications and can benefit from pneumatic dilatation eventually followed by endobronchial stenting (11).
- Vocal cord dysfunction: injury to the recurrent laryngeal nerves with vocal cord palsy is possible, but it is usually temporary and it resolves in several months. It can be useful a chin tuck maneuver to improve airway protection from aspiration. Keep attention during the tracheal dissection maneuvers to avoid nerve injury (12).
- The best treatment for complications remains their prevention.
The tumor involvement of the superior vena cava increases the technical difficulty and the risk of complications.
Results
The results of tracheal sleeve pneumonectomy have improved over time and the procedure is relatively safe in experienced centers. The current perioperative mortality rates in selected patients ranges from 7% to 10%, and the morbidity and mortality are very close to standard pneumonectomy.
Mediastinal lymph node involvement is the main prognostic factor: the long-term results for N2/N3 nodal disease remain poor with a 5-year survival of 12–15% (1). If the mediastinal lymph nodes are negative the survival rate reaches 53%. These data highlights the importance of preoperative mediastinal staging (3).
So if the preoperative staging of tumors invading the airway space demonstrates nodal involvement (stage IIIb), there must be questions and doubts about the advantage of this surgery in those patients (see contraindications) (13).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.08.11). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Perrot M, Shields TW, LoCicero J, et al. Tracheal Sleeve Pneumonectomy. In: Shields TW, LoCicero J, Reed CE, et al. editors. General Thoracic Surgery. 7th Edition. Ambler: Lippincott Williams & Wilkins, 2009.
- Grillo HC. Surgery of the trachea and bronchi. London: BC Decker Inc., 2004
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Young-Beyer P, Wilson RS. Anesthetic management for tracheal resection and reconstruction. J Cardiothorac Anesth 1988;2:821-35. [Crossref] [PubMed]
- Ginsberg RJ. Carinal Resection and Sleeve Pneumonectomy Using a Transsternal Oper Tech Thorac Cardiovasc Surg 1998;3:203-16. [Crossref]
- Dartevelle P, Macchiarini P. Techniques of pneumonectomy. Sleeve pneumonectomy. Chest Surg Clin N Am 1999;9:407-17. xi. [PubMed]
- Grillo HC. Carinal reconstruction. Ann Thorac Surg 1982;34:356-73. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. J Thorac Dis 2016;8:S882-S888. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Ai B, Liao Y, Zhang Z, et al. Single-stage bilateral thoracic surgery via a combined VATS and open approach for left central bronchogenic carcinoma with carinal invasion: report of two cases. J Cardiothorac Surg 2015;10:76. [Crossref] [PubMed]
- Mathisen DJ, Morse C. Master Techniques in Surgery: Thoracic Surgery: Lung Resections, Bronchoplasty. Lippincott Williams and Wilkins, 2014.
- Banki F, Wood DE. Techniques of Performing Left Carinal Pneumonectomy. Oper Tech Thorac Cardiovasc Surg 2007;12:194-209. [Crossref]
- Dartevelle PG, Macchiarini P, Chapelier AR. 1986: Tracheal sleeve pneumonectomy for bronchogenic carcinoma: report of 55 cases. Updated in 1995. Ann Thorac Surg 1995;60:1854-5. [Crossref] [PubMed]
Cite this article as: Nosotti M, Righi I, Damarco F, Rosso L. Tracheal sleeve pneumonectomy: indications and surgical operative techniques. Shanghai Chest 2017;1:29.