Surgical approaches for bronchopleural fistula
Introduction
Bronchopleural fistula (BPF) is defined as a direct communication between the bronchus and the pleural space. BPF can be classified as central, which are fistulous connections between the trachea or a lobar bronchus and the pleural space, or peripheral, which are fistulous connections between the distal airway (segmental bronchi or lung parenchyma) and the pleural space.
In the mid 1950s to early 1960s, the vast majority of BPFs were secondary to pulmonary tuberculosis infection. As drug therapy for tuberculosis has improved, this complication has considerably decreased (1). Currently, complications during bronchopulmonary procedures are the leading cause of BPF despite refinements in surgical techniques and surgical equipment (2-4). Postpneumonectomy BPF is a severe postoperative complication with high rates of morbidity and mortality (5,6). Postlobectomy BPF, however, occurs in less than 1% of patients and has a much lower mortality rate (1,7,8). More rarely, benign pathologies, such as necrotizing pneumonia, empyema, tuberculosis, aspergillosis, granulomatosis with polyangiitis, rheumatologic conditions and pulmonary sarcoidosis, can cause BPF (9).
The clinical suspicion of BPF obligates an urgent and objective investigation that allows proper decision-making for the management of this highly morbid complication. Despite all medical and technical advances, BPF remains a challenge to the thoracic surgeon. In this chapter we will discuss the management of central BPF.
Presentation
Modern lung surgery relies on staplers to transect and seal the bronchus. This strategy has decreased the likelihood of an early postoperative BPF as compared with the hand-sewn techniques used previously. Bronchial fistulas are detected most frequently between the 1st week and 3rd month postoperatively (10) with a peak in incidence between the 8th and 12th postoperative days (8,11). The diagnosis of BPF can be a clinical challenge, but a combination of imaging techniques and invasive procedures, such as bronchoscopy with or without surgical exploration, are used to define the location and the clinical presentation of BPF.
The clinical presentation of a BPF can be acute, subacute, or chronic depending on the timing of surgery (Table 1). This differentiation is important in selecting the proper treatment. In an acute case due to a massive air leak, the patient will present with a tension pneumothorax and subcutaneous emphysema. If the chest tube still in place, an important air leak will be seen at the water seal (12). Sudden expectoration of purulent sputum, acute cough, dyspnea, and mediastinal and tracheal shifts are also common findings. In postpneumonectomy patients, an acute decrease in the pleural liquid level is the common presentation (13). The subacute and chronic clinical presentations of BPF are usually associated with an infected pleural space (empyema) and present with more insidious symptoms with productive cough, fever, leukocytosis, and progressive clinical deterioration with varying levels of respiratory compromise (13).
Table 1
| Acute |
| Tension pneumothorax |
| Subcutaneous emphysema |
| Sudden expectoration of purulent sputum |
| Acute cough |
| Dyspnea |
| Mediastinal and tracheal shifts |
| Acute decrease of the pleural liquid level |
| Subacute and chronic |
| Empyema |
| Productive cough |
| Fever |
| Leukocytosis |
| Progressive clinical deterioration |
BPF, bronchopleural fistula.
Risk factors
There are several known clinical risk factors for postoperative BPF including the patient’s clinical condition preoperatively and the technical features of the surgical procedure performed (Table 2). Diabetes mellitus and chronic obstructive pulmonary disease (COPD) are independent risk factors for postoperative BPF (14,15). Age (>70 years), anemia, adult respiratory distress syndrome, poor nutrition, hypoalbuminemia, systemic use of steroids, empyema, neoadjuvant therapy with chemotherapy or radiation, and tracheostomy have also been described as risk factors for BPF (8,16-23).
Table 2
| Systemic |
| Poor nutrition |
| Sepsis |
| Immunosuppression |
| Diabetes mellitus |
| COPD |
| Anemia |
| Local |
| Neoadjuvant therapy |
| Poor blood supply |
| Active infection |
| Long bronchial stump |
| Extended lymph node dissection |
| Residual or recurrent cancer at the bronchial stump |
| Devascularization of bronchial stump |
| Other |
| Postoperative mechanical ventilation |
| Steroid use |
| Right pneumonectomy |
| Bronchial stump by hand-sewn |
| Tracheostomy |
| Smoking |
BPF, bronchopleural fistula; COPD, chronic obstructive pulmonary disease.
Technical mistakes that can ultimately result in a BPF include a long bronchial stump, disrupted bronchial blood supply, inadequate stump closure, and extended bronchial resection (24). Residual carcinoma at the bronchial stump also increases the chances of BPF (25). BPF is 4 to 5 times more likely after right pneumonectomy as compared with left pneumonectomy (20,26). It is not clear why postpneumonectomy fistulas are more common on the right side than on the left. However, the decreased mediastinal tissue coverage of the right bronchial stump and lack of a dual blood supply to the right main bronchus may contribute to the increased incidence of BPF after right pneumonectomy. Therefore, coverage of the bronchial stump with a well-vascularized flap is strongly suggested (27). Additionally, postoperative ventilator assistance can affect stump healing and the incidence of dehiscence (17,18,20).
Investigation
When investigating a possible BPF, the objective is to estimate the size and precise location of the BPF, understand its relationship to adjacent mediastinal structures, and identify any secondary complications.
Bronchoscopy
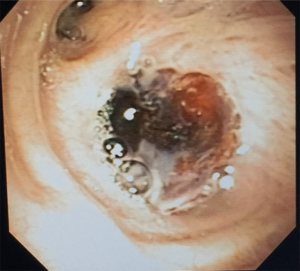
Bronchoscopy is essential for BFP diagnosis and therapeutic planning and can establish the location and size of a BPF with precision (Figure 1). Definitive treatment can sometimes be administered through an endobronchial approach.
Chest radiography
A chest X-ray is the simplest and easiest test to investigate a BPF. The presence of increased intrapleural air space, the appearance of an air-fluid level, and the development of a tension pneumothorax are classical findings of BPF (10,28). A new or larger pneumothorax—commonly noted as a decrease in the air-fluid level, a shift of the mediastinum contralateral to the surgical side, and subcutaneous emphysema—is suggestive of a BPF and warrants a thorough investigation (29). The diagnosis of BPF is an exclusion diagnosis related to postoperative pleural fluid shifts (30). Rarely, a decrease in the air-fluid level in the postpneumonectomy cavity will not be associated with BPF.
Chest computed tomography (CT)
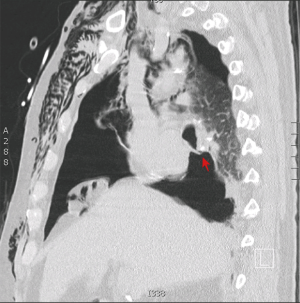
A chest CT scan should be acquired with intravenous contrast to define the anatomic relationship of the fistula with adjacent vasculature, mediastinal structures, pleural space, and the diaphragm (Figure 2). A chest CT can also identify peripheral rind enhancement and air-fluid levels suggestive of localized or multi-loculated empyema (9) (Figure 3).

Virtual bronchoscopy
Virtual bronchoscopy is a 3-dimensional reconstruction of 2-dimensional, helical CT images that provides a simulated, noninvasive, intraluminal tracheo-bronchial evaluation (9). This test essentially allows a complete view of the airway beyond any obstruction. Virtual bronchoscopy can be used prior to endobronchial procedures such as bronchial stent placement. Acute angles in the tracheo-bronchial tree can be studied extensively when planning stent deployment and when evaluating airway complications after stent placement (31,32). Virtual bronchoscopy can also be a useful method for monitoring the healing process (33,34) and evaluating the internal diameter of the bronchial stump prior to the application of sealants.
Nuclear medicine
Nuclear medicine can be considered when CT scans and bronchoscopy cannot identify or sufficiently characterize a fistula (35,36). In one study of ventilation scintigraphy in 28 postpneumonectomy patients, this nuclear medicine technique using 99 m Tc-diethylenetriamine penta-acetate aerosol had a sensitivity of 78% and a specificity of 100% for the detection of BPF, with 86% accuracy (35).
Management
Preventative steps
To reduce the risk of BPF development, the majority of the studies recommend prophylactic bronchial stump coverage in patients with known preoperative risk factors for fistula. The benefit of prophylactic bronchial stump coverage has not been proved by randomized trials, and some of the data in the literature is controversial. In a recent meta-analysis, the incidence of BPF in patients considered at high risk for BPF who received bronchial stump coverage was only slightly higher as compared with patients considered at low risk for BPF who did not undergo prophylactic stump coverage (6.3% vs. 4.0%) (37). In another study, Sfyridis and colleagues randomized patients with diabetes mellitus who underwent pneumonectomy to either resection with reinforcement of the bronchial stump with an intercostal muscle flap or to a conventional resection. The patients who received an intercostal muscle flap had a lower incidence of BPF as compared with the patients who underwent conventional resection (0% vs. 17.1%) (38).
Initial management
Patients with BPF are at high risk of bronchial aspiration, aspiration pneumonia, acute respiratory distress syndrome, and mortality (3,23,39). Protecting the contralateral lung from spillage of pleural fluid is the single most important action when BPF is suspected (9). It is then imperative to rapidly diagnose and manage the BPF.
Appropriate management of a BPF depends on the type of fistula and the clinical condition of the patient. In undernourished patients with chronic BPF and empyema, nutritional rehabilitation has to start before planning a definitive fistula repair (40). Conversely, in patients with acute fistulas, rapid intervention to close the bronchial stump and obliterate the residual pleural space should be performed (1,41,42). Independent of the timing of the BPF, broad-spectrum antibiotics, aggressive nutritional supplementation, and adequate pleural drainage are key to recovery, because almost 80% of patients with BPF have pleural empyema (43).
Noninvasive treatment
On occasion, conservative treatment with antibiotics, nutritional support and proper pleural drainage may allow spontaneous closure of BPF. This noninvasive option is simple; however, the safety of the patent must be ensured. It is particularly useful in patients with postlobectomy fistulas when there is minimal residual pleural space and the air leak progressively decreases (1).
Patching a BPF
BPF are frequently patched with a muscle flap. The principles for a complete closure include (I) antibiotics for pleural sterilization; (II) completed drainage of the pleural space before reoperation; (III) preserving the integrity of the blood supply to the chest wall muscles; (VI) wide debridement and removal of all granulation tissue; (V) identification and closure of the fistula; and (VI) filling the residual space with a vascularized flap (44-47). Several anatomic structures have been used to patch BPFs (Table 3). The serratus anterior and the latissimus dorsi muscles have been described in the treatment of infected residual spaces (1,61-66), with or without BPF (52) without significant addition of morbidity (67). However, use of these muscles as a patch is contraindicated if the muscle was previously divided (15,68).
Table 3
| Anatomic structure | Comments |
|---|---|
| Azygous vein | Used in rare cases |
| Diaphragm | A diaphragmatic flap can be used to reduce the pleural cavity by elevating the remaining diaphragm (48-50) |
| Used in rare cases | |
| Epicardial fat | Used in rare cases |
| Intercostal muscle | One of the most used muscle grafts due to its wide availability, simplicity to harvest, good vascularization, and length |
| A single intercostal flap or multiple intercostal flaps may be used to reinforce a fistula suture and fill the residual space (51) | |
| Latissimus dorsi | Excellent option |
| A bulky muscle that is easy to harvest | |
| Blood supply is from the thoracodorsal artery | |
| One of the most frequently used flaps due to its ability to protect the stump and fill the residual space (52,53) | |
| Omentum | A good choice for the obliteration of the fistula and the pleural space due to its bulkiness and optimal vascular supply |
| The omentum is passed through the substernal space. Due to its singular vascular supply, great care must be taken not to twist the omentum when entering the pleural cavity (54-59) | |
| Parietal pleura | Used in rare cases |
| Pectoralis major | A particularly good option |
| Dual vascularization from the internal mammary and the thoracoabdominal artery (60) | |
| Rectus abdominis | Excellent vascular muscle flap |
| Bilateral option | |
| Vascularization from the inferior epigastric artery | |
| Serratus anterior | Small and thin muscle |
| Vascularization from the lateral thoracic artery | |
| Easy utilization and insertion in the pleural cavity (61) |
Management of the pleural space
When caring for patients with BPF or attempting to avoid BPF in high-risk patients, reduction of the pleural space enables resolution of any empyema and facilitates the closure of small peripheral BPFs. Several procedures have been described to manage the pleural space including pleural tenting, pneumoperitoneum, phrenic nerve block, the Clagett procedure, and open-window thoracotomy (69,70).
Pleural tenting
During the history of general thoracic surgery, many methods have been tried to prevent residual air spaces after thoracic surgery, especially after lung resection for inflammatory lung disease. Pleural tenting is an old method, first described by Miscall and colleagues (71) and Hansen (72), that has recently regained popularity (73-75). This technique is used to reduce the apical pleural space and requires dissection of the parietal pleura off the endothoracic fascia, from the thoracotomy incision towards the apex of the chest cavity. The mobilized parietal pleura are then tented over the residual lung by suturing the border of the pleura to the lower edge of the thoracotomy (72). Importantly, chest tubes should to be placed under the tent. A recent meta-analysis defined pleural tenting as simple and easy to perform. Also, tenting did not prolong the duration of the operation and was not associated with greater morbidity as compared with upper lobectomy without tenting (76).
Pneumoperitoneum
Pneumoperitoneum is the injection of air into the abdominal cavity, causing an elevation of the hemi-diaphragm and thus reducing the residual pleural space postoperatively. In 1924, Reich and colleagues first described the use of pneumoperitoneum for patients with emphysema (77); Carter and colleagues later confirmed the safety of the procedure (78). The main indications for institution of pneumoperitoneum are postoperative residual pleural space, incomplete lung re-expansion after empyema decortication, and severe pleural space problems in adult patients (79). It is also indicated as an adjunct to block major air leaks. Pneumoperitoneum is a safe and effective procedure (79,80) performed with the aid of imaging exams to guide the introduction of air. A catheter is left in place to progressively inject air in the abdomen (79-81).
Phrenic nerve block
Phrenic nerve block is a simple technique that allows temporary reduction of the pleural cavity by raising the dome of the diaphragm. The phrenic nerve block is performed with bupivacaine (0.5%) without epinephrine at the neurovascular pedicle at the level of the pericardium (82). The effect usually lasts 24 hours. This procedure is contraindicated in patients with limited lung function (82).
Clagett procedure
In 1963, Clagett and Geraci (61) described a technique for the management of postpneumonectomy empyema. The technique was a 2-stage procedure: open pleural drainage for control of the septic cavity and closure of BPF and then obliteration of the pleural cavity with antibiotic solution. The Clagett procedure has been reported to be effective in 88% of patients, with failures resulting from persistent or recurrent BPF (83). In an attempt to address these failures, Pairolero and Arnold (84) described the transposition of a well-vascularized extrathoracic muscle as an intermediate step. This modification of the “Clagett” procedure was designed to further reinforce the bronchial stump and to decrease the size of the pleural cavity.
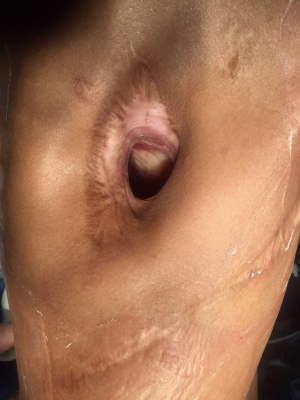
Open-window thoracotomy
The open-window thoracostomy (Figure 4) is an ideal method for draining the septic pleural cavity in patients with empyema after a pulmonary resection (61,85,86), especially in patients with a postpneumonectomy BPF with empyema (87). Open-window thoracostomy was first described by Robinson in 1916 (88) in patients with nontuberculous empyema, and was subsequently revised in 1935 by Eloesser (89) for patients with tuberculous empyema. The Eloesser procedure added the resection of 2-to-3 rib segments and the creation of a skin flap used to epithelialize the entryway in the pleural space. The window should be placed, low and anterior in the chest to facilitate drainage (89).
The timing of attempting to close the window created during open-window thoracostomy is usually dictated by the condition of the pleural cavity and the prognosis of the patient (90). When the pleural space is clean, as characterized by the presence of healthy granulation tissue, it can be closed (66). There is some controversy on the timing for closing the window, but in most cases closure is warranted ~6 months after thoracostomy (90).
Endoscopic treatment
History and indications
Although it is primarily used for diagnostic purposes, flexible bronchoscopy has been gaining ground as a therapeutic modality in patients with BPF and has evolved to treat central BPF, primarily after lobectomy and less frequently after pneumonectomy. In 1983, Roksvaag and colleagues (91) reported two cases of postpneumonectomy BPF closure with synthetic tissue glue applied endoscopically. Several other cases have been reported since then, and endoscopic techniques are considered a safe and feasible alternative to open surgery for management of BPF (92,93).
Despite the fact that surgery is the standard of care for a central BPF when properly indicated, a less invasive approach can be a bridge to a definitive treatment in fragile patients. The primary objective of endoscopic BPF closure is to reduce a potentially life-threatening air leak, prevent aspiration of pleural fluid, and decrease secondary pleural contamination. Once the patient’s clinical condition and nutritional status improve definitive surgical treatment can be attempted (13). Eventually endoscopic treatment can potentially result in long-term BPF closure and complete empyema resolution without requiring permanent-tube thoracostomy or open pleural drainage (92).
Different techniques through flexible or rigid bronchoscopes are available and can be performed under conscious sedation or general anesthesia. These approaches mainly differ in the material used to close the fistula. Success rates reported in retrospective series range widely—from 22.5% to 96.9% (94). This might indirectly represent the lack of standard practices in the endoscopic management of BPF.
Fistula size
Fistula size is one factor that determines whether an endoscopic approach is an appropriate treatment. Smaller fistulas are more suitable for endoscopic closure (92,94,95). In a retrospective series of 35 patients who underwent bronchoscopic repair of BPFs after pneumonectomy, repair was successful in 92.3% of patients with BPFs ≤2 mm in size, 71.4% for BPF >2 and <3 mm, 80% for BPF >3 and <6 mm, and only 33.3% in patients with BPF >6 mm (96). The BPFs ≤2 mm in size were treated with mechanical abrasion causing local inflammation. BPF >2 and <3 mm were treated with submucosal injection of polidocanol; BPF >3 and <6 mm were treated with n-butyl-2-cyanoacrylate glue (Histoacryl) instillation. BPF >6 mm were treated via insertion of an expandable substance filled with fluid n-butyl-2-cyanoacrylate glue to occlude the fistula (94). Before applying any endoscopic therapy, it is advisable to clear secretions and debris from the bronchial stump (97). Mucosa necrosis has been described as an indication for surgical treatment (94).
Biological glues
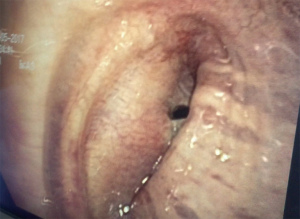
Several types of biological glues with different components have been used for endoscopic BPF closure including fibrin-based glues, cyanoacrylate-based glues (e.g., Histoacryl, TissueSeal, Ann Arbor MI), and albumin-glutaraldehyde tissue adhesive (BioGlue, BioLife Inc., Kennesaw, GA) (96,98-100). Regardless of the glue type, the application technique is very similar. A catheter (e.g., Fogarty no5) is inserted through the working channel of a flexible bronchoscope and placed just above the fistula. The glue is then injected into the fistula, and after a few seconds, it creates a plug that occludes the fistula (Figure 5). Alternatively, some authors (96) have injected the glue in the submucosa with a 21G needle in favor of a theoretically more effective closure due to less glue displacement. An air-leak interruption through the chest tube should be immediately observed. Close clinical and endoscopic surveillance is important for identification of failure to plug the fistula. The need for repeated endoscopic glue applications for successful closure is not uncommon (91). Following mechanical occlusion, the glue induces scar tissue formation, which helps achieve long-term BPF closure (101).
The application of biological glues has some positive aspects, such as (I) potentially providing an instantaneous closure of the BPF, which plays a key role in preventing the development of pleural space infection or treating an empyema that is already onset and (II) using a tool (biological glue) that is already widely applied on humans in a variety of procedures with low risks of complications.
Sclerosing agents
Endoscopic submucosal injection of sclerosing substances or direct application of these agents over the mucosa of the bronchial stump can be used to close BPF by promoting local edema and tissue-healing processes (93,102). Silver nitrate causes cellular damage, because it burns the superficial layer and induces local inflammation and scar tissue formation. The application of sclerosing agents requires the expertise of a skilled endoscopist to avoid injury to surrounding tissues. It can be applied through rigid or flexible bronchoscopy (103). Stratakos and colleagues (104) reported an 81.8% success rate using this technique in a small series of 11 patients with postpneumonectomy or postlobectomy BPF ≤5 mm in size. They applied melted silver nitrate through a flexible bronchoscope until blanching and edema of the mucosa appeared around the fistula. The procedure was repeated up to 10 times at 5- to 7-day intervals until effective sealing was achieved. Boudaya and colleagues (102) successfully treated 16 of 17 patients with postoperative BPFs, mostly ≤5 mm (a success rate of 94.1%). Liquid silver nitrate (1%) was applied around the stump mucosa under direct vision with a catheter through the working channel of a flexible bronchoscope. A solution of 100% carbolic acid has also been used with similar technique, mechanism of action and results for BPF with a median diameter of 4.5 mm (105).
Video-assisted thoracic surgery (VATS)
Several reports have described video-assisted surgery for the closure of BPF. The main benefits of VATS are better visualization, which allows more secure fixation of the vascularized grafts, and a decreased need for intervention as compared with the open approaches discussed below.
Open surgical treatment of BPF
Dehiscence of the bronchial stump after anatomic resection remains the most common cause of BPF (9). Therefore, prevention through complete preoperative evaluation and safe decision-making during surgery is the best way to avoid this complication (11). Standard procedures to reduce the likelihood of BPF in patients at risk include ensuring an appropriate vascular supply to the stump, creating a bronchial stump <1 cm long, and prophylactically covering the bronchial stump with a vascularized flap (1).
Treatment principles
Only a small percentage of BPF will spontaneously close, typically BPF that occur after anatomic segmentectomy or lobectomy where the residual pleural space is small in patients who are healthy otherwise (1). BPF is habitually accompanied by empyema, which can range from exudative pleural effusion to a more chronic fibrinopurulent effusion. So ideally, the repair of the fistula should be performed once the pleural cavity is clean. Ignoring this rule will most certainly result in poor outcome (61,65).
Early BPF
Initial management of a BPF that occurs early after surgery (within 2 weeks) is dependent on drainage of the pleural space with a large chest tube (32F or higher) and positioning the patient in the reverse Trendelenburg position with the affected side down to prevent compromising the contralateral lung. Fluid cultures must be requested, and broad-spectrum antibiotics should be started immediately and continued until culture results are available to dictate a more specific antibiotic range (84).
Early postoperative stump leaks require urgent intervention. After initial bedside management is initiated, an individualized strategy must be planned, and a surgical procedure must take place after the infection is under control (106). Classical surgical management consists of thoracotomy (opening of the previous incision), inspection and searching for the leaking site, debridement of fibrin and infected pleural residue, irrigation of the cavity with antibiotic solution, resection of necrotic tissue around the stump, reclosure with non-absorbable sutures, and reinforcement of the suture with a vascularized pedicle patch (1,82). The most commonly used vascularized patches are derived from the intercostal muscles, serratus anterior, latissimus dorsi, pectoralis major, omental flap, or rectus abdominalis. If a long stump is noticed, it must be resected to its origin and restapled, if possible. Pleurostomy may be performed depending on the size of the fistula, state of infection of the cavity or in poor surgical candidates.
Late BPF
Late postoperative BPF (2 weeks and more after surgery) may require a longer course of treatment. After initial drainage, definitive treatment should be delayed until the infection is under control and the nutritional status of the patient is optimal. Several techniques have been described for the treatment of a BPF occurring late after surgery, ranging from open thoracostomy (Eloesser Flap) to the Clagett or modified Clagett procedure (61,65).
The Clagett procedure, a two-step procedure, begins with open pleural drainage (through an open-window thoracostomy) and resuturing of the bronchial stump followed by packing the space one or two times a day with dressings imbibed with quarter-strength Dakin’s solution or povidone-iodine solution diluted 20:1. After the cavity is judged clean, a second stage takes place that consists of the filling the pleural space with an antiseptic solution (Dab’s solution—0.5 g of neomycin, 0.1 g of polymyxin B sulfate and 80 mg of gentamicin per liter of saline) followed by water-tight wound shutting (61). Zaheer and colleagues demonstrated a rate of success of over 80% with this technique (6).
A transsternal transpericardial approach for BPF repair was initially described by Abruzzini in 1961 and is an option that avoids entering a previous manipulated cavity (107). A full sternotomy is used to access the anterior pericardium, which is then opened. The pulmonary artery is mobilized, allowing opening of the posterior pericardium, and finally, exposure of the carina. The bronchial stump is identified, divided, and closed. A vascularized patch of pericardium is then used to reinforce the suture. Ginsberg and collaborators reported successful management in 77% of patients in their series using this approach (108).
Conclusions
In modern thoracic surgery, the incidence of BPF has substantially decreased due to improvements in preoperative evaluation and surgical techniques. However, BPF still represents a major complication in thoracic surgery that demands proper and accurate management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.06.01). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper WA, Miller JI Jr. Management of bronchopleural fistula after lobectomy. Semin Thorac Cardiovasc Surg 2001;13:8-12. [Crossref] [PubMed]
- Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary resections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg 2000;17:106-10. [Crossref] [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [PubMed]
- al-Kattan K, Cattalani L, Goldstraw P. Bronchopleural fistula after pneumonectomy with a hand suture technique. Ann Thorac Surg 1994;58:1433-6. [Crossref] [PubMed]
- Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: a binational long-term study. J Thorac Cardiovasc Surg 2008;136:179-85. [Crossref] [PubMed]
- Zaheer S, Allen MS, Cassivi SD, et al. Postpneumonectomy empyema: results after the Clagett procedure. Ann Thorac Surg 2006;82:279-86; discussion 286-7. [Crossref] [PubMed]
- Nagahiro I, Aoe M, Sano Y, et al. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann 2007;15:45-8. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Gaur P, Dunne R, Colson YL, et al. Bronchopleural fistula and the role of contemporary imaging. J Thorac Cardiovasc Surg 2014;148:341-7. [Crossref] [PubMed]
- Kirsh MM, Rotman H, Behrendt DM, et al. Complications of pulmonary resection. Ann Thorac Surg 1975;20:215-36. [Crossref] [PubMed]
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [Crossref] [PubMed]
- McManigle JE, Fletcher GL, Tenholder MF. Bronchoscopy in the management of bronchopleural fistula. Chest 1990;97:1235-8. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. [Crossref] [PubMed]
- Li SJ, Zhou XD, Huang J, et al. A systematic review and meta-analysis-does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery? J Thorac Dis 2016;8:1625-38. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. [Crossref] [PubMed]
- Khan JH, Rahman SB, McEihinney DB, et al. Management strategies for complex bronchopleural fistula. Asian Cardiovasc Thorax Ann 2000;8:78-84. [Crossref]
- Darling GE, Abdurahman A, Yi QL, et al. Risk of a right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg 2005;79:433-7. [Crossref] [PubMed]
- Hubaut JJ, Baron O, Al Habash O, et al. Closure of the bronchial stump by manual suture and incidence of bronchopleural fistula in a series of 209 pneumonectomies for lung cancer. Eur J Cardiothorac Surg 1999;16:418-23. [Crossref] [PubMed]
- Deschamps C, Bernard A, Nichols FC 3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72:243-7; discussion 248. [Crossref] [PubMed]
- Wright CD, Wain JC, Mathisen DJ, et al. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg 1996;112:1367-71. [Crossref] [PubMed]
- Smith GH. Complications of cardiopulmonary surgery. 1 edition. London, UK: Bailliere Tindall, 1984:18-20.
- Li S, Fan J, Zhou J, et al. Residual disease at the bronchial stump is positively associated with the risk of bronchoplerual fistula in patients undergoing lung cancer surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;22:327-35. [Crossref] [PubMed]
- Gudbjartsson T, Gyllstedt E, Pikwer A, et al. Early surgical results after pneumonectomy for non-small cell lung cancer are not affected by preoperative radiotherapy and chemotherapy. Ann Thorac Surg 2008;86:376-82. [Crossref] [PubMed]
- Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: an effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg 2005;79:284-8. [Crossref] [PubMed]
- Piccione W, Faber LP. Management of complications related to pulmonary resection. In: Waldhausen JA, Orringer MB. editors. Complications in Cardiothoracic Surgery. St. Louis, MO, Mosby Year-Book, 1991:336-53.
- Lauckner ME, Beggs I, Armstrong RF. The radiological characteristics of bronchopleural fistula following pneumonectomy. Anaesthesia 1983;38:452-6. [Crossref] [PubMed]
- Merritt RE, Reznik SI, DaSilva MC, et al. Benign emptying of the postpneumonectomy space. Ann Thorac Surg 2011;92:1076-81; discussion 1081-2. [Crossref] [PubMed]
- Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol 2008;191:1576-80. [Crossref] [PubMed]
- Nair A, Godoy MC, Holden EL, et al. Multidetector CT and postprocessing in planning and assisting in minimally invasive bronchoscopic airway interventions. Radiographics 2012;32:E201-32. [Crossref] [PubMed]
- McAdams HP, Palmer SM, Erasmus JJ, et al. Bronchial anastomotic complications in lung transplant recipients: virtual bronchoscopy for noninvasive assessment. Radiology 1998;209:689-95. [Crossref] [PubMed]
- Shitrit D, Valdsislav P, Grubstein A, et al. Accuracy of virtual bronchoscopy for grading tracheobronchial stenosis: correlation with pulmonary function test and fiberoptic bronchoscopy. Chest 2005;128:3545-50. [Crossref] [PubMed]
- Mark JB, McDougall IR. Diagnosis and localization of bronchopulmonary air leaks using ventilation scintigraphy. Chest 1997;111:286-9. [Crossref] [PubMed]
- Nielsen KR, Blake LM, Mark JB, et al. Localization of bronchopleural fistula using ventilation scintigraphy. J Nucl Med 1994;35:867-9. [PubMed]
- Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Sfyridis PG, Kapetanakis EI, Baltayiannis NE, et al. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg 2007;84:967-71. [Crossref] [PubMed]
- Hollaus PH, Lax F, el-Nashef BB, et al. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg 1997;63:1391-6; discussion 1396-7. [Crossref] [PubMed]
- Puskas JD, Mathisen DJ, Grillo HC, et al. Treatment strategies for bronchopleural fistula. J Thorac Cardiovasc Surg 1995;109:989-95; discussion 995-6. [Crossref] [PubMed]
- Allen MS, Deschamps C, Trastek VF, et al. Bronchopleural fistula. Chest Surg Clin 1992;823.
- Westcott JL, Volpe JP. Peripheral bronchopleural fistula: CT evaluation in 20 patients with pneumonia, empyema, or postoperative air leak. Radiology 1995;196:175-81. [Crossref] [PubMed]
- Wain JC. Management of late postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am 1996;6:529-41. [PubMed]
- Anderson TM, Miller JI Jr. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg 1995;60:729-33. [Crossref] [PubMed]
- Pairolero PC, Arnold PG. Intrathoracic transfer of flaps for fistulas, exposed prosthetic devices, and reinforcement of suture lines. Surg Clin North Am 1989;69:1047-59. [Crossref] [PubMed]
- Pairolero PC, Trastek VF, Allen MS. Empyema and bronchopleural fistula. Ann Thorac Surg 1991;51:157-8. [Crossref] [PubMed]
- The use of diaphragm grafts for plastic operations in thoracic surgery. J Thorac Cardiovasc Surg 1961;41:348-55. [PubMed]
- Westaby S, Shepherd MP, Nohl-Oser HC. The use of diaphragmatic pedicle grafts for reconstructive procedures in the esophagus and tracheobronchial tree. Ann Thorac Surg 1982;33:486-90. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Early closure of the postpneumonectomy bronchopleural fistula by pedicled diaphragmatic flaps. Ann Thorac Surg 1995;60:714-5. [Crossref] [PubMed]
- Hollaus PH, Huber M, Lax F, et al. Closure of bronchopleural fistula after pneumonectomy with a pedicled intercostal muscle flap. Eur J Cardiothorac Surg 1999;16:181-6. [Crossref] [PubMed]
- Chan EC, Lee TW, Ng CS, et al. Closure of postpneumonectomy bronchopleural fistula by means of single, perforator-based, latissimus dorsi muscle flap. J Thorac Cardiovasc Surg 2002;124:1235-6. [Crossref] [PubMed]
- Abolhoda A, Wirth GA, Bui TD, et al. Harvest technique for pedicled transposition of latissimus dorsi muscle: an old trade revisited. Eur J Cardiothorac Surg 2008;33:928-30. [Crossref] [PubMed]
- Yokomise H, Takahashi Y, Inui K, et al. Omentoplasty for postpneumonectomy bronchopleural fistulas. Eur J Cardiothorac Surg 1994;8:122-4. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Andreetti C, et al. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg 2009;88:212-5. [Crossref] [PubMed]
- Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125:526-32. [Crossref] [PubMed]
- Levashev YN, Akopov AL, Mosin IV. The possibilities of greater omentum usage in thoracic surgery. Eur J Cardiothorac Surg 1999;15:465-8. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Vlahakes GJ, et al. The omentum in the management of complicated cardiothoracic problems. J Thorac Cardiovasc Surg 1988;95:677-84. [PubMed]
- Miller JD, DeHoyos A. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. The University of Toronto and Washington University Lung Transplant Programs. J Thorac Cardiovasc Surg 1993;105:247-52. [PubMed]
- Kalweit G, Feindt P, Huwer H, et al. The pectoral muscle flaps in the treatment of bronchial stump fistula following pneumonectomy. Eur J Cardiothorac Surg 1994;8:358-62. [Crossref] [PubMed]
- Park JS, Eom JS, Choi SH, et al. Use of a serratus anterior musculocutaneous flap for surgical obliteration of a bronchopleural fistula. Interact Cardiovasc Thorac Surg 2015;20:569-74. [Crossref] [PubMed]
- Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg 1963;45:141-5. [PubMed]
- Abrashanoff X. Plastische Methode des Schliessung von Fistelga¨ngen, welche von inneren Organen kommen. Zentralbl Chir 1911;38:186-91.
- Robinson S. The treatment of chronic non-tuberculous empyema. Collected Papers Mayo Clin 1915;7:618-44.
- Arnold PG, Pairolero PC. Intrathoracic muscle flaps. An account of their use in the management of 100 consecutive patients. Ann Surg 1990;211:656-60; discussion 660-2. [Crossref] [PubMed]
- Pairolero PC, Arnold PG, Trastek VF, et al. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 1990;99:958-66; discussion 966-8. [PubMed]
- Deschamps C, Pairolero PC, Allen MS, et al. Management of postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am 1996;6:519-27. [PubMed]
- Widmer MK, Krueger T, Lardinois D, et al. A comparative evaluation of intrathoracic latissimus dorsi and serratus anterior muscle transposition. Eur J Cardiothorac Surg 2000;18:435-9. [Crossref] [PubMed]
- Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg 2000;120:270-5. [Crossref] [PubMed]
- Miller JI Jr. Acute and delayed space problems following pulmonary resection. Chest Surg Clin N Am 1996;6:615-21. [PubMed]
- Rice TW, Kirby TJ. Prolonged air leak. Chest Surg Clin North Am 1992;2:803-11.
- Miscall L, Duffy RW, Nolan RB, et al. The pleural tent as a simultaneous tailoring procedure in combination with pulmonary resection. Am Rev Tuberc 1956;73:831-52. [PubMed]
- Hansen JL. Parietal pleurolysis (the pleural tent) as a simultaneous space-reducing procedure in combination with pulmonary resection. Acta Chir Scand 1957;112:485-8. [PubMed]
- Robinson LA, Preksto D. Pleural tenting during upper lobectomy decreases chest tube time and total hospitalization days. J Thorac Cardiovasc Surg 1998;115:319-26; discussion 326-7. [Crossref] [PubMed]
- Okur E, Kir A, Halezeroglu S, et al. Pleural tenting following upper lobectomies or bilobectomies of the lung to prevent residual air space and prolonged air leak. Eur J Cardiothorac Surg 2001;20:1012-5. [Crossref] [PubMed]
- Brunelli A, Al Refai M, Monteverde M, et al. Pleural tent after upper lobectomy: a randomized study of efficacy and duration of effect. Ann Thorac Surg 2002;74:1958-62. [Crossref] [PubMed]
- Uzzaman MM, Daniel Robb J, Mhandu PC, et al. A meta-analysis assessing the benefits of concomitant pleural tent procedure after upper lobectomy. Ann Thorac Surg 2014;97:365-72. [Crossref] [PubMed]
- Reich L. Der Einfluss des Pneumoperitoneums auf das Lungen-emphysem. Wien Arch Finn Med 1924;8:245-60.
- Carter MG, Gaensler EA, Kyllonen A. Pneumoperitoneum in the treatment of pulmonary emphysema. N Engl J Med 1950;243:549-58. [Crossref] [PubMed]
- Podgaetz E, Berger J, Small J, et al. Therapeutic Pneumoperitoneum: Relevant or Obsolete in 2015? Thorac Cardiovasc Surg 2016; [Epub ahead of print]. [PubMed]
- Cerfolio RJ, Holman WL, Katholi CR. Pneumoperitoneum after concomitant resection of the right middle and lower lobes (bilobectomy). Ann Thorac Surg 2000;70:942-6; discussion 946-7. [Crossref] [PubMed]
- Nielsen KT, Lund L, Larsen LP, et al. Duration of postoperative pneumoperitoneum. Eur J Surg 1997;163:501-3. [PubMed]
- Murthy SC. Air leak and pleural space management. Thorac Surg Clin 2006;16:261-5. [Crossref] [PubMed]
- Clagett OT. Changing aspects of the etiology and treatment of pleural empyema. Surg Clin North Am 1973;53:863-6. [Crossref] [PubMed]
- Pairolero PC, Arnold PG. Bronchopleural fistula: treatment by transposition of pectoralis major muscle. J Thorac Cardiovasc Surg 1980;79:142-5. [PubMed]
- Lemmer JH, Botham MJ, Orringer MB. Modern management of adult thoracic empyema. J Thorac Cardiovasc Surg 1985;90:849-55. [PubMed]
- Shamji FM, Ginsberg RJ, Cooper JD, et al. Open window thoracostomy in the management of postpneumonectomy empyema with or without bronchopleural fistula. J Thorac Cardiovasc Surg 1983;86:818-22. [PubMed]
- Goldstraw P. Treatment of postpneumonectomy empyema: the case for fenestration. Thorax 1979;34:740-5. [Crossref] [PubMed]
- Robinson S. The Treatment of chronic non-tuberculous chronic emyeme. Surg Gynecol Obstet 1912;22:257.
- Eloesser L. Of an operation for tuberculous empyema. Ann Thorac Surg 1969;8:355-7. [Crossref] [PubMed]
- García-Yuste M, Ramos G, Duque JL, et al. Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg 1998;65:818-22. [Crossref] [PubMed]
- Roksvaag H, Skalleberg L, Nordberg C, et al. Endoscopic closure of bronchial fistula. Thorax 1983;38:696-7. [Crossref] [PubMed]
- Hollaus PH, Lax F, Janakiev D, et al. Endoscopic treatment of postoperative bronchopleural fistula: experience with 45 cases. Ann Thorac Surg 1998;66:923-7. [Crossref] [PubMed]
- Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Scappaticci E, Ardissone F, Ruffini E, et al. As originally published in 1994: Postoperative bronchopleural fistula: endoscopic closure in 12 patients. Updated in 2000. Ann Thorac Surg 2000;69:1629-30. [Crossref] [PubMed]
- Hamid UI, Jones JM. Closure of a bronchopleural fistula using glue. Interact Cardiovasc Thorac Surg 2011;13:117-8. [Crossref] [PubMed]
- Sippel JM, Chesnutt MS. Bronchoscopic therapy for bronchopleural fistula. J Bronchol 1998;5:61-9. [Crossref]
- Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Lang-Lazdunski L. Closure of a bronchopleural fistula after extended right pneumonectomy after induction chemotherapy with BioGlue surgical adhesive. J Thorac Cardiovasc Surg 2006;132:1497-8. [Crossref] [PubMed]
- Ranu H, Gatheral T, Sheth A, et al. Successful endobronchial seal of surgical bronchopleural fistulas using BioGlue. Ann Thorac Surg 2009;88:1691-2. [Crossref] [PubMed]
- Menard JW, Prejean CA, Tucker WY. Endoscopic closure of bronchopleural fistulas using a tissue adhesive. Am J Surg 1988;155:415-6. [Crossref] [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [Crossref] [PubMed]
- Høier-Madsen K, Schulze S, Møller Pedersen V, et al. Management of bronchopleural fistula following pneumonectomy. Scand J Thorac Cardiovasc Surg 1984;18:263-6. [Crossref] [PubMed]
- Stratakos G, Zuccatosta L, Porfyridis I, et al. Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg 2009;138:603-7. [Crossref] [PubMed]
- Wang Z, Yu HB, Luo Q, et al. Treatment of Bronchopleural Fistula with Carbolic Acid instilled through Bronchofiberscope in post-pulmonectomy patients. J Cardiothorac Surg 2015;10:120. [Crossref] [PubMed]
- Zanotti G, Mitchell JD. Bronchopleural Fistula and Empyema After Anatomic Lung Resection. Thorac Surg Clin 2015;25:421-7. [Crossref] [PubMed]
- Abruzzini P. Tratamento chirurgico delle fistulae del broncho principale consecutive pneumonectomia tubercolosi. Chir Thorac 1961;14:165-71.
- Ginsberg RJ, Pearson FG, Cooper JD, et al. Closure of chronic postpneumonectomy bronchopleural fistula using the transsternal transpericardial approach. Ann Thorac Surg 1989;47:231-5. [Crossref] [PubMed]
Cite this article as: Dal Agnol G, Vieira A, Oliveira R, Ugalde Figueroa PA. Surgical approaches for bronchopleural fistula. Shanghai Chest 2017;1:14.