支气管胸膜瘘的手术治疗
前言
支气管胸膜瘘 (BPF) 是指支气管和胸膜腔之间直接连通,可分为中央型和外周型,中央型指气管或叶支气管与胸膜腔之间通过瘘管相连接,外周型指远端气道(段支气管或肺实质)和胸膜腔之间形成瘘道。
在20世纪50年代中期至60年代初期,绝大多数BPF继发于肺结核感染。随着抗结核药物及内科治疗措施的不断发展,这种并发症已大大减少[1]。目前,外科手术技术和器械均有较大提升和改进,但支气管肺相关手术仍然是BPF发生的主要原因[2-4]。全肺切除术后的BPF是一种严重并发症,发病率和死亡率都很高[5-6],而肺叶切除术后BPF的发生率不到1%,而且患者死亡率要相对低得多[7-8]。另外,还有某些良性病变,如坏死性肺炎、脓胸、肺结核、曲霉病、肉芽肿伴多血管炎、风湿病和肺结节病等,术后发生BPF的概率则更低[9]。对于临床怀疑高度BPF的患者,为了做出正确的治疗决策,降低死亡率,需要临床医生尽快进行相关的、有针对性的辅助检查。尽管医学和技术都取得了进步,但BPF仍然是胸外科医生面临的挑战。本文将讨论中央型BPF的预防和治疗。
介绍
现代肺外科手术依靠切割闭合器来切断、闭合支气管,与以前手工缝合技术相比,降低了术后早期BPF的可能性。BPF通常发生在手术后第1周~3个月之间,术后第8~12天发生瘘的概率最高[8,11]。BPF的诊断具有一定挑战性,通过影像学检查和侵入性操作相结合,如支气管镜检查加或不加手术探查,可用于明确BPF的位置和临床表现。
BPF根据手术时间和临床表现可分为急性、亚急性或慢性(表1),这种分类方式对治疗的选择非常重要。因残端大量漏气而导致的急性期患者,往往会出现张力性气胸和皮下气肿,如果胸管位置良好,会看到胸管有严重的持续性漏气[12]。此外,术后突然出现的刺激性咳嗽伴咳脓痰、呼吸困难、纵隔和气管移位也是BPF早期常见的临床表现,而在全肺切除术患者中,术后胸膜腔积液量明显下降是最典型的临床表现。BPF的亚急性和慢性期的临床表现通常和胸膜腔的感染程度(脓胸)有关,其临床表现更加隐匿,主要包括咳痰、发热、白细胞增多和不同程度的呼吸系统损害的临床症状[13]。

Full table
危险因素
术后形成BPF的危险因素主要包括患者术前的一般情况和外科手术方式两个方面(表2)。糖尿病和慢性阻塞性肺疾病(COPD)是术后发生BPF的独立危险因素[14,15]。年龄>70岁、贫血、成人呼吸窘迫综合征、营养不良、低蛋白血症、全身使用类固醇激素、脓胸、新辅助放化疗及气管切开术也被认为是导致BPF发生的危险因素[8,16-23]。

Full table
支气管残端过长、缺血、闭合不全和支气管扩大切除[24]、残端癌残留等术中不当处理均会增加BPF的发生概率[25]。右全肺切除术后BPF的发生率是左全肺的4~5倍,目前尚不清楚其发生的主要机制[20,26],可能和右侧支气管残端纵隔组织覆盖率低,右主支气管缺乏双重血供有关,因此,有专家建议使用血供良好的带蒂组织瓣覆盖支气管残端[27]。此外,术后呼吸机辅助通气也会影响支气管残端愈合、增加残端裂开的发生率[17,18,20]。
辅助检查
在临床工作中,如果高度怀疑BPF时,需要通过进一步检查来明确BPF的大小和位置、了解其与相邻纵隔结构的毗邻关系,以及是否会导致相关并发症的发生。
纤维支气管镜检查
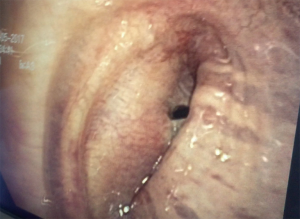
支气管镜检查对于BPF诊断和治疗至关重要,它能明确BPF的位置和大小(图1),也可以行支气管镜下的介入治疗。
胸部X线检查
胸部X线检查是明确有无BPF最简单、有效的检查方式之一,BPF的典型的影像学表现是胸膜腔内气体增加、出现液气平面和张力性气胸[10,28]。如临床上出现气液平下降、纵膈向对侧移位和皮下气肿,往往提示存在BPF,需要做更进一步的检查[29]。BPF与术后胸腔积液减少的关系密切[30],但在极少数情况下,肺切除术后腔内气液水平的降低与BPF无关。
胸部CT检查
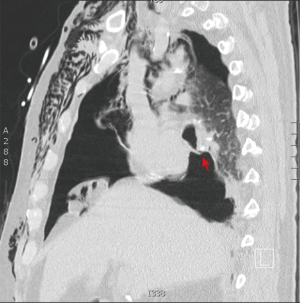
通过胸部CT增强检查,可以明确瘘口位置及其与相邻血管、纵隔结构、胸膜腔和膈肌的解剖关系(图2)。胸部CT还可以通过脓腔外侧缘组织是否存在强化及是否存在气液平面,来诊断局限性或多发分隔的脓胸[9](图3)。
虚拟支气管镜检查
虚拟支气管镜检查是在二维螺旋CT图像基础上的三维重建成像,可提供模拟、无创、腔内气管–支气管评估[9]。该检查可以在没有任何阻塞的情况下获得完整气道三维视图,也可用于支气管内手术,如放置支气管支架,气管–支气管树的夹角可以在术前规划和术后评估支架位置后,以及评估是否有气道并发症时进行研究[31,32]。它也可用于在应用封堵剂之前评估支气管残端内径,同时监测愈合过程[33,34]。
核医学检查
当CT扫描和支气管镜检查均无法明确是否存在瘘口时,可以考虑核医学检查[35,36]。在一项对28名肺切除术后患者进行通气显像的研究中,使用99mTc–二亚乙基三胺五乙酸盐气雾剂作为介质,检测BPF的灵敏性为78%,特异性为100%,准确性为86%35。
预防和治疗
预防措施
为了降低BPF发生率,大多数学者建议对已知合并BPF相关危险因素的患者进行预防性支气管残端覆盖,虽然在相关临床随机对照试验中,尚未证明预防性支气管残端覆盖的益处,同时试验中的部分数据也存在争议。在最近的一项荟萃分析中,接受支气管残端覆盖的高危患者中,BPF发生率要略高于未接受预防性残端覆盖的低危患者(6.3% vs 4.0%)37。在另一项研究中,Sfyridis等[38]将患有糖尿病同时接受全肺切除术的患者进行随机分组,一组用肋间肌瓣加固支气管残端,另一组进行支气管常规切除、闭合术,与常规治疗组相比,接受肋间肌瓣加固的患者BPF的发生率更低(0 vs 17.1%)。
初步治疗
BPF患者发生误吸性肺炎、急性呼吸窘迫综合征和死亡的风险均很高[3,23,39]。当临床怀疑BPF时,保护对侧肺免于胸腔积液倒灌而发生误吸是最重要的一项预防措施[9],因此快速诊断、早期治疗对BPF至关重要。
BPF的治疗取决于胸膜瘘的类型和患者的全身情况,对于中晚期BPF合并慢性脓胸且存在严重营养不良患者,必须先进行有效营养支持之后,才能进行BPF的修补[40]。但对于急性期的患者,应尽快关闭支气管残端并消除感染胸膜残腔,从而获得满意的治疗效果[1,41,42]。无论是那种类型的BPF,广谱抗生素、积极的营养支持、充分的胸腔引流都是治疗的关键,因为约80%的BPF患者合并存在脓胸[43]。
保守治疗
一部分患者,通过抗感染、营养支持和恰当的胸腔引流等创伤较小的保守治疗措施,BPF可自行闭合,特别是对于肺叶切除术后,残腔较小且漏气逐渐减少的BPF的患者尤为适用[1]。
BPF修补
带蒂肌瓣常用来修补BPF,手术成功的先决条件包括——(Ⅰ)胸膜腔用抗生素进行灭菌处理;(Ⅱ)再次手术前需保证胸膜腔已充分引流; (Ⅲ) 胸壁外肌肉组织的血供没有被破坏; (Ⅳ) 扩大清创和去除所有肉芽组织; (Ⅴ) 瘘口的识别和闭合; (Ⅵ) 用带血管的肌皮瓣填充剩余残腔[44-47]。多种解剖结构可用于修补BPF(表3),前锯肌和背阔肌被报道可以用于治疗伴或不伴BPF的感染性残腔[1,61-66],并且没有增加并发症的发病率[67]。但如果这些肌肉组织在既往手术中被切断过,则无法被用于BPF的修补[15,68]。

Full table
胸膜间隙的管理
缩小胸膜腔几乎适用于所有脓胸,包括BPF患者的治疗或在高危患者中预防BPF发生,此外,促进外周型BPF的愈合也很关键。主要手术方法包括:胸膜帐篷化、气腹、膈神经阻滞、Clagett手术和胸壁开窗术[69,70]。
胸膜帐篷化
在胸外科手术的发展过程中,临床医生尝试了许多方法来处理胸外科术后、特别是感染性疾病行肺切除术后的残腔,胸膜帐篷化由Miscall等[71]和Hansen[72]首次提出,它是一种古老的手术方法,但最近又重新开始流行起来[73-75]。这种技术可以缩小胸膜顶的间隙,具体手术操作是沿胸壁切口上缘切开、游离壁层胸膜至胸腔顶,然后将分离的胸膜边缘与切口下缘缝合,活动的壁层胸膜覆盖在余肺之上,还有重要的一点,胸引管应放置于帐篷下方[72]。最近的一项荟萃分析表明,胸膜帐篷化手术操作简单,与传统上叶切除手术相比,并不会明显增加手术时间及并发症发生率[76]。
气腹
气腹是将空气注入腹腔,使膈肌位置抬高,从而减少术后胸膜腔间隙。1924年,Reich等[77]首次提出用气腹来治疗肺气肿,Carter等[78]后来证实了该手术的安全性。气腹手术的主要适应证包括:手术后残腔存在、胸膜剥脱术后肺复张不完全和严重的胸膜腔疾病[79]。它也被看作是严重持续性漏气的补充治疗,在现代影像学技术的引导下,通过留置导管,逐步向腹腔注入空气[79-81],这是一种非常安全、有效的治疗方法[79,80]。
膈神经阻滞
膈神经阻滞是一项相对简单的操作技术,主要通过暂时性的抬高膈肌来缩小胸膜腔。具体做法是在心包水平、神经血管根部使用不含肾上腺素的0.5%布比卡因进行膈神经阻滞,其作用效果通常可以持续24小时,但这种方法不能用于有限制性通气功能障碍的患者[82]。
Clagett手术
1963年,Clagett和Geraci[61]描述了一种处理肺切除术后脓胸的方法,主要分为两步。首先通过开放引流来控制残腔内感染、关闭BPF,然后用抗生素溶液填充封闭胸膜腔。据报道,Clagett手术的有效率可以达到88%,失败的原因主要是持续性或复发性BPF[83]。为了解决这一问题,Pairolero和Arnold[84]增加了将血供良好的胸外带蒂肌瓣转移至残腔这一步骤,这种改良的Clagett术式可以更进一步加固支气管残端并减小胸膜腔的体积。
胸壁开窗术
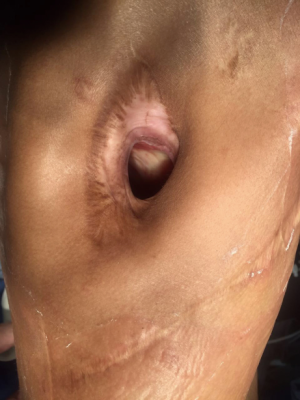
胸壁开窗术(图4)是一种肺切除术后严重感染化脓胸膜腔较为理想的引流方法[61,85,86],特别是全肺切除术后BPF合并脓胸的患者[87]。该术式最早于1916年由Robinson提出并运用于在非结核性脓胸的患者,Eloesser在1935年将其用于结核性脓胸患者并进行了适当改良,Eloesser术式建议切除2~3根部分肋骨,建立一个覆盖于胸膜腔入口的皮瓣,同时开窗位置最好位于胸壁前、下方,以便于引流[89]。
胸壁开窗和闭合的手术时机主要取决于胸膜腔的感染控制情况和患者的预后[90]。如果胸膜腔内肉芽组织新鲜,则表明腔内是干净的,可以进行闭合[66]。关于关闭胸壁开窗的时间仍存在一些争议,在大多数病例中,关闭时间一般选在开胸术6个月之后[90]。
内镜治疗
历史和适应证
纤维支气管镜既往主要用于诊断,但现在它已经成为BPF的一种重要治疗手段,包括肺叶切除术后中央型BPF,但较少应用于全肺切除术后的BPF。1983年,Roksvaag等[91]首次报道内镜下应用合成组织胶成功封堵2例肺切除术后的BPF,从那之后,又陆续有相关病例报道,纤支镜技术被认为是治疗BPF的一种安全、有效的选择[93]。
尽管外科手术是中央型BPF的标准治疗方法,但对于一般情况差的患者,微创治疗可以作为手术治疗的桥梁。纤支镜下BPF闭合的主要目的是减少存在生命威胁的严重漏气,防止胸腔积液误吸,减少继发性胸腔感染,当患者的临床情况和营养状况得到改善后,再尝试行外科手术治疗[13]。同时,内镜治疗也可能使BPF长期闭合、脓胸彻底得到控制,而不需要行永久性胸廓造口或开放式胸膜腔引流[92]。
在镇静或全身麻醉下,有支气管软镜和硬镜的两种不同技术均可供选择,主要是根据封堵材料不同来决定。在诸多回顾性研究报道中,封堵成功率的差异较大,成功率为22.5%~96.9%[94],这也间接反映出BPF的内镜治疗缺乏统一的标准。
瘘口直径
内镜治疗是否合适主要取决于BPF的瘘口直径,越小的瘘口越适合于内镜下闭合[92,94,95]。在一项回顾性研究中,共计35例肺切除术后BPF接受内镜下修补瘘口的患者,BPF≤2 mm的患者成功率为92.3%,2 mm<BPF≤3 mm的患者成功率为71.4%,3 mm<BPF≤6 mm的患者成功率为80%,而BPF>6 mm的患者成功率只有33.3%。BPF≤2 mm的患者主要采用物理刺激来引起局部炎症,2 mm<BPF≤3 mm的患者予黏膜下注射聚多卡醇(硬化剂),3mm<BPF≤6mm的患者采用正丁基-2-氰基丙烯酸酯胶(组织胶水)局部滴注,BPF>6mm的患者通过填充正丁基-2-氰基丙烯酸酯胶的可膨胀介质来阻塞瘘管。同时,在内镜治疗之前,建议清理支气管残端的分泌物和碎片组织[97],黏膜坏死也是手术指征之一[94]。
生物胶
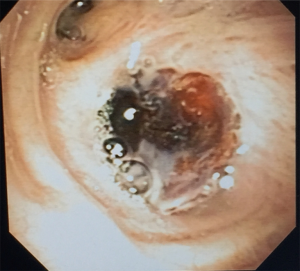
内镜下BPF闭合可以选择多种不同成分的生物胶,包括纤维蛋白胶,丙烯腈胶(Histoacryl, tissue seal, Ann Arbor MI)和白蛋白戊二醛组织胶(biogglue, BioLife Inc.,Kennesaw, GA)[96,98-100]。但无论哪种介质,其技术原理基本相似,都是通过Fogarty no 5导管从支气管镜的工作通道进入,在瘘管的正上方分多次将胶水注射到瘘口内(图5)。另一种方法是,用21G针头在黏膜下层注射胶水,由于胶水很少移位,理论上可以有效地封闭瘘口[96]。注射完成后,应立即通过胸管观察漏气情况,临床表现和内镜下观察瘘口是否完全封闭均非常关键,有些患者需要反复多次内镜下的注射才能有效封堵[91],在实现物理闭塞后,胶水可诱导瘢痕组织形成,从而实现BPF永久闭合[101]。生物胶的应用对BPF的治疗起了积极的促进作用,主要体现在以下两方面:①使BPF的暂时性的关闭成为可能; ② 生物胶这种生物介质被广泛使用于人体各类手术中,并发症小。
硬化剂
内镜下将硬化剂直接注射到瘘口周围黏膜下层组织,通过局部水肿和组织愈合关闭BPF[93,102],如硝酸银能造成细胞损伤,灼伤表层组织,诱发局部炎症和疤痕形成。支气管黏膜下注射硬化剂需要非常专业、操作熟练的内镜医生在纤维软镜下完成,最大程度减小对周围正常组织的损伤[103]。Stratakos等[104]报道了在11例全肺或肺叶切除术后BPF大小≤5mm的患者,将融化的硝酸银通过纤支镜注射到黏膜下,直到瘘口周围的组织出现漂白样改变和水肿,重复10次,每次间隔5~7天,直到瘘口被成功封闭,成功率可达81.8%。Boudaya等[102]则将1%硝酸银溶液通过纤支镜的工作通道直接应用于残端周围黏膜,成功治愈了17例肺切除术后BPF患者中的16例,成功率为94.1%,其中大部分患者的BPF≤5 mm。100%石碳酸溶液也被用于直径≤4.5 mm的BPF,其操作方法和作用机制与硝酸银类似[105]。
胸腔镜手术
有报道描述了用胸腔镜辅助手术来治疗BPF,与接下来要讨论的开放手术相比,VATS手术的主要优点是具备更好的视野,可以使带血管蒂的移植组织得到更安全、有效的固定,减少一些非必要的损伤。
外科手术治疗
解剖肺切除后支气管残端裂开仍然是形成BPF最常见的原因[9],因此,完整的术前评估和术中正确处理决策是避免这一并发症的最佳方法[11]。确保支气管残端有良好的血供、合适长度、预防性的使用带血管蒂的组织瓣覆盖残端均可以降低BPF发生率。
治疗原则
只有一小部分肺叶或肺段切除术后发生细支气管肺泡胸膜瘘的患者,因其残腔小、一般情况良好可以自行痊愈[1]。而其他BPF通常伴有脓胸存在,可以是渗出性胸腔积液,也可能是脓性积液,理想状态下,瘘口的修补应该在胸膜腔被清理干净后进行,否则可能会导致严重的后果[61,65]。
早期BPF
术后早期BPF(2周内发生)主要处理方式为应用大号胸管(32 F或更粗)行胸腔闭式引流术,患者取头低脚高位,患侧向下,以防止污染健侧肺,同时立即使用广谱抗生素,反复、多次进行脓液培养,根据培养结果选择更敏感的抗生素[84]。
术后早期残端瘘在经过紧急处理之后,必须制定个体化治疗策略,手术也需要在感染基本得到控制后[106]。经典的外科治疗措施包括再次开胸手术(原切口进胸),仔细检查并寻找瘘口,清除胸腔内纤维素和感染坏死组织,抗生素溶液冲洗胸腔,用不可吸收缝合线重新缝合残端,最后用带血管蒂的自体组织瓣加固残端[1,82]。最常用的自体组织瓣通常来源于肋间肌、前锯肌、背阔肌、胸大肌、大网膜瓣或腹直肌。如果发现支气管残端过长,则需将残端切除至正常范围,是否行胸膜切除术需要根据瘘口的大小、腔内感染的情况或手术条件差的情况决定。
晚期BPF
术后晚期BPF(发生时间超过2周)可能需要较长的治疗周期,在初期引流后,下一阶段的治疗方案需要等到患者感染得到控制、营养状况改善至最佳。前文已总结了几种治疗术后晚期BPF的方法,从开胸(Eloesser皮瓣)到Clagett或改良Clagett手术[61,65]。
Clagett手术分为两步,首先是胸腔开放引流(通过胸壁开窗),然后修复支气管残端,用四分之一强度达金溶液或聚维酮碘溶液稀释后的纱布填充,每天换药1~2次,在确定脓腔基本干净后,再进行第二阶段手术,用抗菌溶液(达金溶液:0.5 g新霉素,0.1 g硫酸多粘菌素B和80mg庆大霉素/每升生理盐水)填充胸膜腔,然后关闭切口[61],Zaheer等[6]证实这种手术方式的成功率超过80%。
Abruzzini[107]在1961年首次提出经胸骨与经心包膜入路修复BPF,这种手术方式可以避免进入既往手术过的污染腔隙。手术采用正中切口,劈开胸骨,纵行切开心包,在肺动脉上方打开心包后壁,显露出气管隆凸,分离出支气管残端并重新闭合,然后用带血管蒂的心包补片缝合加固。Ginsberg等[108]在其研究中发现,使用这种方法治疗BPF的成功率达到77%。
总结
在现代胸外科手术中,随着术前评估和手术技术的不断改进和完善,BPF的发生率已大大降低。然而,BPF仍然是胸外科手术的主要并发症之一,需要及时、有效的治疗。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.06.01). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper WA, Miller JI Jr. Management of bronchopleural fistula after lobectomy. Semin Thorac Cardiovasc Surg 2001;13:8-12. [Crossref] [PubMed]
- Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary resections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg 2000;17:106-10. [Crossref] [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [PubMed]
- al-Kattan K, Cattalani L, Goldstraw P. Bronchopleural fistula after pneumonectomy with a hand suture technique. Ann Thorac Surg 1994;58:1433-6. [Crossref] [PubMed]
- Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: a binational long-term study. J Thorac Cardiovasc Surg 2008;136:179-85. [Crossref] [PubMed]
- Zaheer S, Allen MS, Cassivi SD, et al. Postpneumonectomy empyema: results after the Clagett procedure. Ann Thorac Surg 2006;82:279-86; discussion 286-7. [Crossref] [PubMed]
- Nagahiro I, Aoe M, Sano Y, et al. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann 2007;15:45-8. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Gaur P, Dunne R, Colson YL, et al. Bronchopleural fistula and the role of contemporary imaging. J Thorac Cardiovasc Surg 2014;148:341-7. [Crossref] [PubMed]
- Kirsh MM, Rotman H, Behrendt DM, et al. Complications of pulmonary resection. Ann Thorac Surg 1975;20:215-36. [Crossref] [PubMed]
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [Crossref] [PubMed]
- McManigle JE, Fletcher GL, Tenholder MF. Bronchoscopy in the management of bronchopleural fistula. Chest 1990;97:1235-8. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Li SJ, Fan J, Zhou J, et al. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg 2016;102:328-39. [Crossref] [PubMed]
- Li SJ, Zhou XD, Huang J, et al. A systematic review and meta-analysis-does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery? J Thorac Dis 2016;8:1625-38. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. [Crossref] [PubMed]
- Khan JH, Rahman SB, McEihinney DB, et al. Management strategies for complex bronchopleural fistula. Asian Cardiovasc Thorax Ann 2000;8:78-84. [Crossref]
- Darling GE, Abdurahman A, Yi QL, et al. Risk of a right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg 2005;79:433-7. [Crossref] [PubMed]
- Hubaut JJ, Baron O, Al Habash O, et al. Closure of the bronchial stump by manual suture and incidence of bronchopleural fistula in a series of 209 pneumonectomies for lung cancer. Eur J Cardiothorac Surg 1999;16:418-23. [Crossref] [PubMed]
- Deschamps C, Bernard A, Nichols FC 3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72:243-7; discussion 248. [Crossref] [PubMed]
- Wright CD, Wain JC, Mathisen DJ, et al. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg 1996;112:1367-71. [Crossref] [PubMed]
- Smith GH. Complications of cardiopulmonary surgery. 1 edition. London, UK: Bailliere Tindall, 1984:18-20.
- Li S, Fan J, Zhou J, et al. Residual disease at the bronchial stump is positively associated with the risk of bronchoplerual fistula in patients undergoing lung cancer surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;22:327-35. [Crossref] [PubMed]
- Gudbjartsson T, Gyllstedt E, Pikwer A, et al. Early surgical results after pneumonectomy for non-small cell lung cancer are not affected by preoperative radiotherapy and chemotherapy. Ann Thorac Surg 2008;86:376-82. [Crossref] [PubMed]
- Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: an effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg 2005;79:284-8. [Crossref] [PubMed]
- Piccione W, Faber LP. Management of complications related to pulmonary resection. In: Waldhausen JA, Orringer MB. editors. Complications in Cardiothoracic Surgery. St. Louis, MO, Mosby Year-Book, 1991:336-53.
- Lauckner ME, Beggs I, Armstrong RF. The radiological characteristics of bronchopleural fistula following pneumonectomy. Anaesthesia 1983;38:452-6. [Crossref] [PubMed]
- Merritt RE, Reznik SI, DaSilva MC, et al. Benign emptying of the postpneumonectomy space. Ann Thorac Surg 2011;92:1076-81; discussion 1081-2. [Crossref] [PubMed]
- Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol 2008;191:1576-80. [Crossref] [PubMed]
- Nair A, Godoy MC, Holden EL, et al. Multidetector CT and postprocessing in planning and assisting in minimally invasive bronchoscopic airway interventions. Radiographics 2012;32:E201-32. [Crossref] [PubMed]
- McAdams HP, Palmer SM, Erasmus JJ, et al. Bronchial anastomotic complications in lung transplant recipients: virtual bronchoscopy for noninvasive assessment. Radiology 1998;209:689-95. [Crossref] [PubMed]
- Shitrit D, Valdsislav P, Grubstein A, et al. Accuracy of virtual bronchoscopy for grading tracheobronchial stenosis: correlation with pulmonary function test and fiberoptic bronchoscopy. Chest 2005;128:3545-50. [Crossref] [PubMed]
- Mark JB, McDougall IR. Diagnosis and localization of bronchopulmonary air leaks using ventilation scintigraphy. Chest 1997;111:286-9. [Crossref] [PubMed]
- Nielsen KR, Blake LM, Mark JB, et al. Localization of bronchopleural fistula using ventilation scintigraphy. J Nucl Med 1994;35:867-9. [PubMed]
- Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Sfyridis PG, Kapetanakis EI, Baltayiannis NE, et al. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg 2007;84:967-71. [Crossref] [PubMed]
- Hollaus PH, Lax F, el-Nashef BB, et al. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg 1997;63:1391-6; discussion 1396-7. [Crossref] [PubMed]
- Puskas JD, Mathisen DJ, Grillo HC, et al. Treatment strategies for bronchopleural fistula. J Thorac Cardiovasc Surg 1995;109:989-95; discussion 995-6. [Crossref] [PubMed]
- Allen MS, Deschamps C, Trastek VF, et al. Bronchopleural fistula. Chest Surg Clin 1992;823.
- Westcott JL, Volpe JP. Peripheral bronchopleural fistula: CT evaluation in 20 patients with pneumonia, empyema, or postoperative air leak. Radiology 1995;196:175-81. [Crossref] [PubMed]
- Wain JC. Management of late postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am 1996;6:529-41. [PubMed]
- Anderson TM, Miller JI Jr. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg 1995;60:729-33. [Crossref] [PubMed]
- Pairolero PC, Arnold PG. Intrathoracic transfer of flaps for fistulas, exposed prosthetic devices, and reinforcement of suture lines. Surg Clin North Am 1989;69:1047-59. [Crossref] [PubMed]
- Pairolero PC, Trastek VF, Allen MS. Empyema and bronchopleural fistula. Ann Thorac Surg 1991;51:157-8. [Crossref] [PubMed]
- The use of diaphragm grafts for plastic operations in thoracic surgery. J Thorac Cardiovasc Surg 1961;41:348-55. [PubMed]
- Westaby S, Shepherd MP, Nohl-Oser HC. The use of diaphragmatic pedicle grafts for reconstructive procedures in the esophagus and tracheobronchial tree. Ann Thorac Surg 1982;33:486-90. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Early closure of the postpneumonectomy bronchopleural fistula by pedicled diaphragmatic flaps. Ann Thorac Surg 1995;60:714-5. [Crossref] [PubMed]
- Hollaus PH, Huber M, Lax F, et al. Closure of bronchopleural fistula after pneumonectomy with a pedicled intercostal muscle flap. Eur J Cardiothorac Surg 1999;16:181-6. [Crossref] [PubMed]
- Chan EC, Lee TW, Ng CS, et al. Closure of postpneumonectomy bronchopleural fistula by means of single, perforator-based, latissimus dorsi muscle flap. J Thorac Cardiovasc Surg 2002;124:1235-6. [Crossref] [PubMed]
- Abolhoda A, Wirth GA, Bui TD, et al. Harvest technique for pedicled transposition of latissimus dorsi muscle: an old trade revisited. Eur J Cardiothorac Surg 2008;33:928-30. [Crossref] [PubMed]
- Yokomise H, Takahashi Y, Inui K, et al. Omentoplasty for postpneumonectomy bronchopleural fistulas. Eur J Cardiothorac Surg 1994;8:122-4. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Andreetti C, et al. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg 2009;88:212-5. [Crossref] [PubMed]
- Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125:526-32. [Crossref] [PubMed]
- Levashev YN, Akopov AL, Mosin IV. The possibilities of greater omentum usage in thoracic surgery. Eur J Cardiothorac Surg 1999;15:465-8. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Vlahakes GJ, et al. The omentum in the management of complicated cardiothoracic problems. J Thorac Cardiovasc Surg 1988;95:677-84. [PubMed]
- Miller JD, DeHoyos A. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. The University of Toronto and Washington University Lung Transplant Programs. J Thorac Cardiovasc Surg 1993;105:247-52. [PubMed]
- Kalweit G, Feindt P, Huwer H, et al. The pectoral muscle flaps in the treatment of bronchial stump fistula following pneumonectomy. Eur J Cardiothorac Surg 1994;8:358-62. [Crossref] [PubMed]
- Park JS, Eom JS, Choi SH, et al. Use of a serratus anterior musculocutaneous flap for surgical obliteration of a bronchopleural fistula. Interact Cardiovasc Thorac Surg 2015;20:569-74. [Crossref] [PubMed]
- Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg 1963;45:141-5. [PubMed]
- Abrashanoff X. Plastische Methode des Schliessung von Fistelga¨ngen, welche von inneren Organen kommen. Zentralbl Chir 1911;38:186-91.
- Robinson S. The treatment of chronic non-tuberculous empyema. Collected Papers Mayo Clin 1915;7:618-44.
- Arnold PG, Pairolero PC. Intrathoracic muscle flaps. An account of their use in the management of 100 consecutive patients. Ann Surg 1990;211:656-60; discussion 660-2. [Crossref] [PubMed]
- Pairolero PC, Arnold PG, Trastek VF, et al. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 1990;99:958-66; discussion 966-8. [PubMed]
- Deschamps C, Pairolero PC, Allen MS, et al. Management of postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am 1996;6:519-27. [PubMed]
- Widmer MK, Krueger T, Lardinois D, et al. A comparative evaluation of intrathoracic latissimus dorsi and serratus anterior muscle transposition. Eur J Cardiothorac Surg 2000;18:435-9. [Crossref] [PubMed]
- Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg 2000;120:270-5. [Crossref] [PubMed]
- Miller JI Jr. Acute and delayed space problems following pulmonary resection. Chest Surg Clin N Am 1996;6:615-21. [PubMed]
- Rice TW, Kirby TJ. Prolonged air leak. Chest Surg Clin North Am 1992;2:803-11.
- Miscall L, Duffy RW, Nolan RB, et al. The pleural tent as a simultaneous tailoring procedure in combination with pulmonary resection. Am Rev Tuberc 1956;73:831-52. [PubMed]
- Hansen JL. Parietal pleurolysis (the pleural tent) as a simultaneous space-reducing procedure in combination with pulmonary resection. Acta Chir Scand 1957;112:485-8. [PubMed]
- Robinson LA, Preksto D. Pleural tenting during upper lobectomy decreases chest tube time and total hospitalization days. J Thorac Cardiovasc Surg 1998;115:319-26; discussion 326-7. [Crossref] [PubMed]
- Okur E, Kir A, Halezeroglu S, et al. Pleural tenting following upper lobectomies or bilobectomies of the lung to prevent residual air space and prolonged air leak. Eur J Cardiothorac Surg 2001;20:1012-5. [Crossref] [PubMed]
- Brunelli A, Al Refai M, Monteverde M, et al. Pleural tent after upper lobectomy: a randomized study of efficacy and duration of effect. Ann Thorac Surg 2002;74:1958-62. [Crossref] [PubMed]
- Uzzaman MM, Daniel Robb J, Mhandu PC, et al. A meta-analysis assessing the benefits of concomitant pleural tent procedure after upper lobectomy. Ann Thorac Surg 2014;97:365-72. [Crossref] [PubMed]
- Reich L. Der Einfluss des Pneumoperitoneums auf das Lungen-emphysem. Wien Arch Finn Med 1924;8:245-60.
- Carter MG, Gaensler EA, Kyllonen A. Pneumoperitoneum in the treatment of pulmonary emphysema. N Engl J Med 1950;243:549-58. [Crossref] [PubMed]
- Podgaetz E, Berger J, Small J, et al. Therapeutic Pneumoperitoneum: Relevant or Obsolete in 2015? Thorac Cardiovasc Surg 2016; [Epub ahead of print]. [PubMed]
- Cerfolio RJ, Holman WL, Katholi CR. Pneumoperitoneum after concomitant resection of the right middle and lower lobes (bilobectomy). Ann Thorac Surg 2000;70:942-6; discussion 946-7. [Crossref] [PubMed]
- Nielsen KT, Lund L, Larsen LP, et al. Duration of postoperative pneumoperitoneum. Eur J Surg 1997;163:501-3. [PubMed]
- Murthy SC. Air leak and pleural space management. Thorac Surg Clin 2006;16:261-5. [Crossref] [PubMed]
- Clagett OT. Changing aspects of the etiology and treatment of pleural empyema. Surg Clin North Am 1973;53:863-6. [Crossref] [PubMed]
- Pairolero PC, Arnold PG. Bronchopleural fistula: treatment by transposition of pectoralis major muscle. J Thorac Cardiovasc Surg 1980;79:142-5. [PubMed]
- Lemmer JH, Botham MJ, Orringer MB. Modern management of adult thoracic empyema. J Thorac Cardiovasc Surg 1985;90:849-55. [PubMed]
- Shamji FM, Ginsberg RJ, Cooper JD, et al. Open window thoracostomy in the management of postpneumonectomy empyema with or without bronchopleural fistula. J Thorac Cardiovasc Surg 1983;86:818-22. [PubMed]
- Goldstraw P. Treatment of postpneumonectomy empyema: the case for fenestration. Thorax 1979;34:740-5. [Crossref] [PubMed]
- Robinson S. The Treatment of chronic non-tuberculous chronic emyeme. Surg Gynecol Obstet 1912;22:257.
- Eloesser L. Of an operation for tuberculous empyema. Ann Thorac Surg 1969;8:355-7. [Crossref] [PubMed]
- García-Yuste M, Ramos G, Duque JL, et al. Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg 1998;65:818-22. [Crossref] [PubMed]
- Roksvaag H, Skalleberg L, Nordberg C, et al. Endoscopic closure of bronchial fistula. Thorax 1983;38:696-7. [Crossref] [PubMed]
- Hollaus PH, Lax F, Janakiev D, et al. Endoscopic treatment of postoperative bronchopleural fistula: experience with 45 cases. Ann Thorac Surg 1998;66:923-7. [Crossref] [PubMed]
- Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Scappaticci E, Ardissone F, Ruffini E, et al. As originally published in 1994: Postoperative bronchopleural fistula: endoscopic closure in 12 patients. Updated in 2000. Ann Thorac Surg 2000;69:1629-30. [Crossref] [PubMed]
- Hamid UI, Jones JM. Closure of a bronchopleural fistula using glue. Interact Cardiovasc Thorac Surg 2011;13:117-8. [Crossref] [PubMed]
- Sippel JM, Chesnutt MS. Bronchoscopic therapy for bronchopleural fistula. J Bronchol 1998;5:61-9. [Crossref]
- Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Lang-Lazdunski L. Closure of a bronchopleural fistula after extended right pneumonectomy after induction chemotherapy with BioGlue surgical adhesive. J Thorac Cardiovasc Surg 2006;132:1497-8. [Crossref] [PubMed]
- Ranu H, Gatheral T, Sheth A, et al. Successful endobronchial seal of surgical bronchopleural fistulas using BioGlue. Ann Thorac Surg 2009;88:1691-2. [Crossref] [PubMed]
- Menard JW, Prejean CA, Tucker WY. Endoscopic closure of bronchopleural fistulas using a tissue adhesive. Am J Surg 1988;155:415-6. [Crossref] [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [Crossref] [PubMed]
- Høier-Madsen K, Schulze S, Møller Pedersen V, et al. Management of bronchopleural fistula following pneumonectomy. Scand J Thorac Cardiovasc Surg 1984;18:263-6. [Crossref] [PubMed]
- Stratakos G, Zuccatosta L, Porfyridis I, et al. Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg 2009;138:603-7. [Crossref] [PubMed]
- Wang Z, Yu HB, Luo Q, et al. Treatment of Bronchopleural Fistula with Carbolic Acid instilled through Bronchofiberscope in post-pulmonectomy patients. J Cardiothorac Surg 2015;10:120. [Crossref] [PubMed]
- Zanotti G, Mitchell JD. Bronchopleural Fistula and Empyema After Anatomic Lung Resection. Thorac Surg Clin 2015;25:421-7. [Crossref] [PubMed]
- Abruzzini P. Tratamento chirurgico delle fistulae del broncho principale consecutive pneumonectomia tubercolosi. Chir Thorac 1961;14:165-71.
- Ginsberg RJ, Pearson FG, Cooper JD, et al. Closure of chronic postpneumonectomy bronchopleural fistula using the transsternal transpericardial approach. Ann Thorac Surg 1989;47:231-5. [Crossref] [PubMed]

伍勇勇
硕士研究生,毕业于上海中医药大学,目前就职于浙江省立同德医院心胸外科。研究生及工作期间参与973项目子课题、国家自然科学基金、浙江省医药卫生科技计划等多项国家级、省级科研项目。以第一作者发表SCI论文3篇,中华及核心期刊数篇。(更新时间:2021/8/12)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Dal Agnol G, Vieira A, Oliveira R, Ugalde Figueroa PA. Surgical approaches for bronchopleural fistula. Shanghai Chest 2017;1:14.