
Solitary mediastinal metastasis from breast cancer after 14 years: case report
Highlight box
Key findings
• This report presents the rare case of solitary mediastinal lymph node metastasis originating from breast cancer. The patient, a 50-year-old female, previously underwent curative mastectomy at 36 years old and robot-assisted modified radical hysterectomy for cancer of the uterine body at 46 years old.
What is known and what is new?
• Breast cancer often causes axillary lymph node metastasis. In advanced breast cancer, lung or mediastinal lymph node metastasis is occasionally detected. However, solitary mediastinal lymph node metastasis is rare.
What is the implication, and what should change now?
• It was unknown whether solitary lymph node metastasis was owing to breast cancer or uterine body cancer. Video-assisted thoracoscopic surgery enabled curative resection and definitive diagnosis, and she had no recurrence for 6 years after surgery. If solitary mediastinal lymph node metastasis was detected, definitive resection may be an alternative method of treatment.
Introduction
Background
Breast cancer often causes axillary lymph node metastasis. In advanced breast cancer, lung or mediastinal lymph node metastasis is occasionally detected. However, mediastinal lymph node metastasis only occurs in approximately 2% of patients with breast cancer undergoing surveillance. Moreover, oligometastasis to the mediastinal lymph node is much rarer (1).
Rationale and knowledge gap
When a solitary swollen mediastinal lymph node is detected in patients with a history of double cancer surgery, we need to rule out its origin being malignant from their prior history of cancer.
Here, we present a case of solitary left middle mediastinal lymph node metastasis 14 years after right breast cancer surgery. We present this article in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-24-9/rc).
Case presentation
A 50-year-old woman was admitted to our hospital because of a solitary mediastinal mass. The patient has a history of right mastectomy and axillary lymph node dissection at age 36 for a 1.8 cm diameter breast cancer located on the right upper inner area. The pathological findings of the resected breast cancer were invasive ductal carcinoma, scirrhous type, t1, n1. The pathological stage was classified as IIA based on the Japanese Classification of Breast Cancer. Immunohistochemical analysis revealed a strong positive for human epidermal growth factor receptor 2 (HER2), and a positive for estrogen receptor (ER) and progesterone receptor (PR). She received postoperative chemotherapy with cyclophosphamide, epirubicin, and fluorouracil. She also received endocrine therapy with tamoxifen. Later, she underwent a robot-assisted modified radical hysterectomy for uterine body cancer at the age of 46 years. The pathological stage was pT1aN0M0, stage Ia, endometrial cancer.
A periodical computed tomography (CT) scan 4 years after uterine body cancer surgery revealed solitary left middle mediastinal lymphadenopathy (Figure 1A,1B). It was located at the fifth level of the thoracic spine, surrounded by the aortic arch, left main pulmonary artery, left upper truncus, and left upper pulmonary vein. The diameter of the lymph node was 3.2 cm × 2.0 cm. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scan was used to detect accumulation of fluorodeoxyglucose in the right axillary lymph node and middle mediastinal lymph node, and accumulation was not detected at any other site. The maximum standard uptake value (SUVmax) of the axillary lymph node was 2.4, whereas the left middle mediastinal lymph node had an SUVmax of 3.4 (Figure 1C). Malignant cells were not detected during the axillary lymph node biopsy.

The mediastinal mass was diagnosed as a metastatic tumor possibly derived from either breast or uterine body cancer. Therefore, we diagnose by resecting the mediastinal mass using video-assisted thoracoscopic surgery (VATS). A 6-cm utility port incision was made in the 4th intercostal space, and two additional ports of 1.5-cm skin incision were made in the 6th intercostal space. A thoracoscopy revealed a prominent, smooth-surfaced tumor in the thoracic cavity, which was carefully dissected (Figure 2). The absence of tumor invasion into vessels permitted complete resection. The postoperative course of the patient was not complicated. The resected specimen was 3.3 cm × 2.5 cm × 2.2 cm in diameter. Immunohistochemical staining of the resected specimen was positive for gross cystic disease fluid protein (GCDFP)-15, which is a breast cancer marker. On the other hand, the tumor tissue was negative for CD5 and terminal deoxynucleotidyl transferase, and those are markers for malignant lymphoma. Finally, the lymph node was diagnosed as metastasis from breast cancer. The biological properties of the metastatic lymph node were ER (+), PgR (−), HER2 (−) (Figure 3).

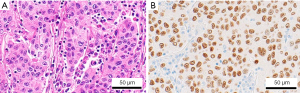
After discharge, she has been receiving endocrine therapy with tamoxifen and LH-releasing hormone agonist (LHRHa). She has not shown any signs of recurrence 6 years after surgery.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Breast cancer often causes axillary lymph node metastasis, particularly in advanced breast cancer, lung or mediastinal lymph node metastasis can occasionally occur, whereas oligometastasis in the mediastinal lymph node is rare (2,3). Solitary metastasis sites of breast cancer have been reported in the thymus, pancreas, lung, subxiphoid region, and brain (4-8). However, solitary mediastinal lymph node metastasis occurs in only 2% of patients with breast cancer undergoing surveillance (1).
In our case, solitary mediastinal tumor was difficult to diagnose because of two reasons. First, the tumor was surrounded by pulmonary arteries and it seemed to be an atypical finding. Second, the patient had experienced breast and uterine body cancer on two occasions. Therefore, we planned to perform a VATS biopsy and definite extirpation if possible. The tumor was adjacent but it did not adhere to the pulmonary artery, so we were able to dissect the pulmonary artery and nerves from the tumor by taping the vessels, and we safely performed definite tumor extirpation.
Solitary mediastinal tumors from breast cancer are rarely reported. Kreisman et al. examined 660 patients with breast cancer over 5 years. Thoracic metastasis was detected in 119 cases (9). There were only ten patients with a solitary initial focus of metastatic disease confined to the thorax, of which two were solitary mediastinal metastasis.
In addition, the mechanism of how the right breast cancer metastasized to the left mediastinal lymph node is also an issue. Generally, the lymphatic flow from breast cancer located inside the mammary gland flows into the right thoracic duct from the parasternal lymph node. In contrast, cancer from outside the mammary gland flows through the pectoralis major muscle into the right thoracic duct via the thoracic lymph node, central lymph node, and subclavian lymph node. Our case is a left mediastinal lymph node metastasis from a right breast cancer arising from the internal mammary gland, which is very rare because the usual lymphatic flow system does not apply. There are reports in the literature of an anomalous thoracic duct that lacks a right thoracic duct and flows into the left thoracic duct (10). Although the precise mechanism is unknown, we considered the possibility that our case also metastasized from the right parasternal lymph node through the left thoracic duct to the left mediastinal lymph node via a network of lymphatic vessels.
As far as we know, there are only a few reports of solitary mediastinal metastatic tumors that were derived from breast cancer (4,11). Moreover, in our survey of articles, we could not identify any other case reports of solitary contralateral middle mediastinal lymph node metastasis of breast cancer.
In our case, solitary mediastinal lymph node metastasis was diagnosed 14 years after mastectomy without another metastasis. Dunnwald et al. reported that women with ER+/PR+ tumors have better prognoses than those with ER+/PR−, ER−/PR+, or ER−/PR− tumors. In our case, the status of hormone receptor ER was positive, PgR was positive, and HER2 was strongly positive. A study suggests that triple-positive status contributes to late recurrence (12).
In our case, it was difficult to make a histopathological diagnosis preoperatively. Tumor resection, the only useful strategy, revealed mediastinal lymph node metastasis of breast cancer.
Conclusions
Solitary mediastinal lymph node metastasis of breast cancer is very rare. According to the literature about mediastinal metastasis of extrathoracic cancer, resection could be done safely, and it is useful to achieve local control of the disease and histopathological diagnosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-24-9/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-24-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-24-9/coif). D.K. serves as an unpaid editorial board member of Shanghai Chest from September 2023 to August 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kamby C, Andersen J, Ejlertsen B, et al. Pattern of spread and progression in relation to the characteristics of the primary tumour in human breast cancer. Acta Oncol 1991;30:301-8. [Crossref] [PubMed]
- Preda L, Rizzo S, Latronico A, et al. Calcified metastatic mediastinal lymph nodes from mucinous breast adenocarcinoma. Eur Radiol 2004;14:1318-9. [Crossref] [PubMed]
- Riquet M, Berna P, Brian E, et al. Intrathoracic lymph node metastases from extrathoracic carcinoma: the place for surgery. Ann Thorac Surg 2009;88:200-5. [Crossref] [PubMed]
- Yamashita T, Watahiki M, Asai K. Mediastinal Metastasis of Breast Cancer Mimicking a Primary Mediastinal Tumor. Am J Case Rep 2020;21:e925275. [Crossref] [PubMed]
- Kitami A, Kamio Y, Gen R, et al. A case of lung cancer with postoperative solitary mediastinal lymph node metastasis from breast cancer. Japanese Journal of Lung Cancer 2009;49:278-81.
- Apodaca-Rueda M, Chaim FHM, Garcia MDS, et al. Solitary pancreatic metastasis from breast cancer: case report and review of literature. Sao Paulo Med J 2019;137:201-5. [Crossref] [PubMed]
- Kitada M, Sato K, Matsuda Y, et al. Role of treatment for solitary pulmonary nodule in breast cancer patients. World J Surg Oncol 2011;9:124. [Crossref] [PubMed]
- Patel SH, Saito YD, Li Z, et al. A solitary brain metastasis as the only site of recurrence of HR positive, HER2 negative breast cancer: a case report and review of the literature. J Med Case Rep 2021;15:4. [Crossref] [PubMed]
- Kreisman H, Wolkove N, Schwartz Finkelstein H, et al. Breast cancer and thoracic metastases: review of 119 patient. Thorax 1983;38:175-9. [Crossref] [PubMed]
- Johnson OW, Chick JF, Chauhan NR, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol 2016;26:2482-93. [Crossref] [PubMed]
- Li J, Wang DD, Zhao YN, et al. Clinical assessment of magnetic resonance imaging-guided radiofrequency ablation for breast cancer. Mol Clin Oncol 2019;11:411-5. [Crossref] [PubMed]
- Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007;9:R6. [Crossref] [PubMed]
Cite this article as: Kimura D, Kaneta A, Muto C, Kimura S, Sasaki T, Tani K, Matsuo T, Hatanaka R, Tsushima T, Minakawa M. Solitary mediastinal metastasis from breast cancer after 14 years: case report. Shanghai Chest 2024;8:19.


