
Comparative efficacy of lobar resection and sublobar resection in patients with stage IA lung invasive mucinous adenocarcinoma: insights from the SEER database with propensity score matching
Highlight box
Key findings
• This study reveals that sublobar resection for stage IA lung invasive mucinous adenocarcinoma (LIMA) patients offers comparable long-term outcomes to lobar resection.
What is known and what is new?
• It is established that the extent of surgical resection impacts outcomes in stage IA LIMA patients.
• This research adds new insights by demonstrating the non-inferiority of sublobar resection compared to lobar resection, challenging previous perceptions.
What is the implication, and what should change now?
• These findings suggest a shift in surgical approach for stage IA LIMA, advocating for a more individualized treatment strategy.
Introduction
Lung cancer remains a significant health concern, holding the dubious distinction of leading in cancer-related mortalities across the globe (1). Among its various histological types, adenocarcinoma stands out as the most prevalent. Within this subset, lung invasive mucinous adenocarcinoma (LIMA) is classified as a distinct variant, previously termed mucinous bronchioloalveolar carcinoma, as per the 2015 World Health Organization (WHO) guidelines (2,3).
Although LIMAs, characterized by goblet and columnar cells with intracytoplasmic mucin, represent only 2–10% of lung adenocarcinomas, their diagnosis presents unique challenges. The inconspicuous cytologic atypia in LIMA cells, their lepidic-predominant growth pattern, and the potential for mixed mucinous presentations can confound accurate diagnosis, especially from limited biopsy specimens (4). On CT images, LIMA typically presents as solid nodules or appears similar to pneumonia. Lee and colleagues noted that only 19 (23%) out of 82 LIMA cases were of the pneumonia type. They further emphasized that these pneumonia-type IMAs were frequently both clinically and pathologically more advanced, generally leading to a less favorable prognosis than the nodular types (5). These challenges are further complicated by the heterogeneous nature of LIMA, with some studies highlighting a non-aggressive nature and better overall survival (OS) compared to non-mucinous adenocarcinomas, while others suggest the contrary (6,7). A previous study analyzed the risk factors for LIMA patients, and found that patients receiving lobar resection are subjected to better survival outcomes compared with sublobar resection (8). What’s more, intrapulmonary relapse, leading to a notably poorer prognosis, was more prevalent in the LIMA patients compared to the other patients with lung adenocarcinoma (6). These observations appear to discourage the use of sublobar resection for LIMA.
Historically, lobar resection has been the gold standard for early-stage lung cancer (9). However, advances in diagnostic precision have rekindled interest in sublobar resection, especially for smaller tumors. Recent years have witnessed the substantial increase of evidence for the equivalence of sublobar resection and lobar resection in early-stage non-small cell lung cancer (NSCLC) (10,11). Notably, the JCOG0802 trial recently demonstrated the efficacy of segmentectomy in clinical stage IA NSCLC, influencing the treatment landscape (12). Additionally, the CALGB 140503 study highlighted that sublobar resection was non-inferior to lobar resection in peripheral NSCLC tumors ≤2 cm, underscoring the significance of tailored approaches (13). However, previous studies have seldomly concentrated the effect of resection extent on the long-term outcomes of patients with certain pathology type, especially the LIMA. Should lobar resection provide a superior prognosis for stage IA LIMA patients compared to sublobar resection, it may be prioritized when the frozen section procedure indicates a LIMA diagnosis. Thus, amidst evolving evidence and treatment paradigms, our study focuses on LIMA patients. Our findings underscore the potential for differentiated surgical strategies based on the specific characteristics of stage IA LIMA, which could lead to more personalized treatment approaches. Clarifying the comparative efficacy of lobar versus sublobar resection could influence future clinical guidelines, promoting tailored surgical interventions that optimize outcomes for patients with this distinct subtype of lung cancer. We present this article in accordance with the STROBE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-24-3/rc).
Methods
Data source
In this research, we gathered patient information from 18 cancer registries that are part of the population-based network. These registries contribute additional treatment-related data to the Surveillance, Epidemiology, and End Results (SEER) database, accessible at http://seer.cancer.gov/. Utilizing the SEER*Stat software (version 8.3.9; seer.cancer.gov/seerstat), we specifically retrieved data regarding individuals diagnosed with lung cancer. Our criteria for data selection included patients with a diagnosis localized to the “Lung and Bronchus” and those identified between the years 2004 and 2016.
Patient screening and exclusion criteria
The selection of patients for this study was guided by specific criteria for inclusion: (I) diagnosis of LIMA [the International Classification of Diseases for Oncology-3 (ICD-O-3) histology code 8253]: invasive mucinous adenocarcinoma and ICD-O-3 code 8480/8481 for mucinous adenocarcinoma; (II) clinical stage of pT1N0M0; (III) age 18 years or older; (IV) lung cancer as the initial primary tumor; (V) tumor size not exceeding 2 cm. On the other hand, the exclusion criteria encompassed: (I) lack of survival duration data; (II) missing information on race, marital status, tumor location, grade, T stage, N stage, and sites of metastasis; (III) absence of lobar or sublobar resection treatment. Following these criteria, the identified cohort was categorized into two groups based on the surgical procedure received: those undergoing lobar resection and those who had sublobar resection.
Variable selection
We extracted several key variables from the SEER database for this analysis. These included demographic details like “age recode with <1 year old”, and “race recode (White, Black, other)”, as well as “sex”. Clinical and pathological characteristics encompassed “primary site-labeled”, “histologic type ICD-O-3”, “grade”, and “laterality”. Staging information was detailed under “derived AJCC stage group”, “derived AJCC T”, “derived AJCC N”, and “derived AJCC M”, for both the 7th [2010–2015] and 6th [2004–2015] editions. Treatment modalities were represented by “RX Summ-Surg Prim Site (1998+)”, “RX Summ-Scope Reg LN Sur (2003+)”, “RX Summ-Surg Oth Reg/Dis (2003+)”, along with “Chemotherapy recode”, and “Radiation recode”. Metastasis at diagnosis was categorized for specific sites such as bone, brain, liver, and lung (2010+). Additional variables included “survival months”, “vital status recode”, “first malignant primary indicator”, and “total number of in situ/malignant tumors for patient”. We also transformed the T and N stages into the AJCC 8th TNM stage, while the M stage remained as per the original classification. Notably, in the 7th AJCC TNM stage, M1b indicated distant metastasis. This category was further subdivided in the 8th edition into M1b (single extrathoracic metastasis) and M1c (multiple extrathoracic metastases).
Outcomes
In our research, we focused on examining both lung cancer-specific survival (CSS) and OS to determine prognostic factors and patient outcomes. The SEER database provided the causes of death, which were determined based on death certificate data. In the context of CSS, patients who died due to causes unrelated to lung cancer were treated as censored at their time of death. Conversely, OS was measured from the initial cancer diagnosis to the date of death, irrespective of the cause. The SEER database is updated yearly, incorporating new data on patient follow-up and survival rates. For the purposes of our study, the most recent update of patient data was in December 2016. Therefore, we calculated survival time as the interval in months from the point of cancer diagnosis to either the date of death or the cut-off date for censored cases, which was December 31, 2016.
Statistical analysis
This study analyzed baseline characteristics of patients undergoing sublobar or lobar resection, using statistical methods for continuous (mean ± standard deviation or median) and categorical variables (frequencies, percentages). Categorical variables were analyzed with Pearson’s χ² or Fisher’s exact test, and continuous ones with the Student’s t-test. We employed propensity score matching (PSM) and stabilized inverse probability of treatment weight (IPTW) to minimize confounding in this observational study. Baseline characteristics were used for PSM and IPTW to estimate unbiased treatment effects which were applied in survival analyses. The PSM was performed using the ‘nearest’ neighbor method with a caliper width of 0.2 standard deviations of the logit of the propensity score to ensure close matches. This process aimed to balance the baseline characteristics across groups, with a 1:1 matching ratio. The covariates included in the matching algorithm were age, sex, year of diagnosis, race, laterality, primary site, grade, radiation therapy, chemotherapy, lymphadenectomy, and the total number of in situ/malignant tumors for each patient. Standardized mean difference (SMD) was calculated to compare the difference before and after PSM.
Survival curves were generated using the Kaplan-Meier method and analyzed with Cox proportional hazards models for various cohorts. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported, considering lobar resection as the reference group. Subgroup analyses included age, sex, tumor grade, and malignancy number. Statistical significance was set at a two-sided P value of 0.05. Data extraction and analyses were performed using SEER*Stat, SPSS, and R software.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was not required for this study as it is a retrospective analysis using de-identified data obtained from the SEER database, which is publicly available and exempt from this requirement.
Results
Basic characteristics
Between January 1, 2004, and December 31, 2016, data on 666,690 individuals diagnosed with lung cancer were collated from the SEER database. Following the application of precise inclusion and exclusion criteria, the final cohort for analysis comprised 947 patients diagnosed with LIMA. The patient selection methodology is delineated in Figure 1. Within this cohort, 596 patients (62.9%) underwent lobar resection, while the remaining 351 (37.1%) were treated with sublobar resection. Table 1 enumerates the core characteristics. The lobar resection group had a significantly lower age, higher proportions of tumors in upper and middle lobe of location, higher No. malignancies (P=0.01 for age, P=0.04 for location, and P<0.001 for No. malignancy). Notably, a greater proportion of patients in the lobar resection group received radiation therapy and lymphadenectomy (both P<0.001). Yet, other baseline characteristics did not exhibit significant disparities between the two groups (P>0.05 for all other variables). Post-PSM, no pronounced difference in foundational characteristics was observed between the groups (all P>0.05). The SMD before and after PSM was depicted in Figure 2, and the SMD before and after IPTW was depicted in Figure S1.
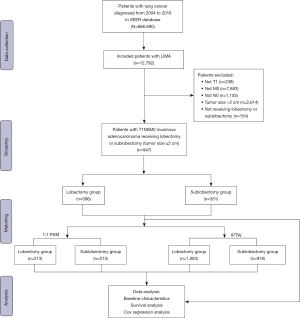
Table 1
| Group | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lobar resection (n=596) | Sublobar resection (n=351) | P | SMD | Lobar resection (n=213) | Sublobar resection (n=213) | P | SMD | ||
| Age, n (%) | 0.01 | 0.241 | 0.15 | 0.252 | |||||
| 20–54 years | 83 (13.9) | 32 (9.1) | 10 (4.7) | 13 (6.1) | |||||
| 55–64 years | 161 (27.0) | 79 (22.5) | 59 (27.7) | 42 (19.7) | |||||
| 65–74 years | 236 (39.6) | 144 (41.0) | 86 (40.4) | 92 (43.2) | |||||
| 75–84 years | 105 (17.6) | 84 (23.9) | 48 (22.5) | 61 (28.6) | |||||
| 85+ years | 11 (1.8) | 12 (3.4) | 10 (4.7) | 5 (2.3) | |||||
| Sex, n (%) | 0.35 | 0.067 | 0.70 | 0.047 | |||||
| Female | 356 (59.7) | 198 (56.4) | 110 (51.6) | 115 (54.0) | |||||
| Male | 240 (40.3) | 153 (43.6) | 103 (48.4) | 98 (46.0) | |||||
| Year of diagnosis, n (%) | 0.74 | 0.113 | 0.94 | 0.108 | |||||
| 2004–2005 | 63 (10.6) | 33 (9.4) | 18 (8.5) | 15 (7.0) | |||||
| 2006–2007 | 86 (14.4) | 41 (11.7) | 21 (9.9) | 21 (9.9) | |||||
| 2008–2009 | 95 (15.9) | 52 (14.8) | 27 (12.7) | 27 (12.7) | |||||
| 2010–2011 | 100 (16.8) | 65 (18.5) | 34 (16.0) | 42 (19.7) | |||||
| 2012–2013 | 119 (20.0) | 73 (20.8) | 49 (23.0) | 46 (21.6) | |||||
| 2014–2015 | 133 (22.3) | 87 (24.8) | 64 (30.0) | 62 (29.1) | |||||
| Race, n (%) | 0.11 | 0.171 | 0.95 | 0.058 | |||||
| Black | 55 (9.2) | 19 (5.4) | 15 (7.0) | 12 (5.6) | |||||
| Other | 45 (7.6) | 20 (5.7) | 7 (3.3) | 7 (3.3) | |||||
| White | 494 (82.9) | 311 (88.6) | 190 (89.2) | 193 (90.6) | |||||
| Unknown | 2 (0.3) | 1 (0.3) | 1 (0.5) | 1 (0.5) | |||||
| Laterality, n (%) | 0.25 | 0.083 | >0.99 | 0.009 | |||||
| Left | 249 (41.8) | 161 (45.9) | 92 (43.2) | 93 (43.7) | |||||
| Right | 347 (58.2) | 190 (54.1) | 121 (56.8) | 120 (56.3) | |||||
| Location, n (%) | 0.04 | 0.213 | 0.85 | 0.113 | |||||
| Lower lobe | 290 (48.7) | 199 (56.7) | 127 (59.6) | 119 (55.9) | |||||
| Lung | 5 (0.8) | 7 (2.0) | 2 (0.9) | 2 (0.9) | |||||
| Middle lobe | 43 (7.2) | 16 (4.6) | 6 (2.8) | 10 (4.7) | |||||
| Upper lobe | 255 (42.8) | 128 (36.5) | 77 (36.2) | 81 (38.0) | |||||
| Overlapping lesion of lung | 3 (0.5) | 1 (0.3) | 1 (0.5) | 1 (0.5) | |||||
| Grade, n (%) | 0.35 | 0.145 | >0.99 | 0.041 | |||||
| Grade I | 337 (56.5) | 199 (56.7) | 123 (57.7) | 121 (56.8) | |||||
| Grade II | 149 (25.0) | 83 (23.6) | 47 (22.1) | 49 (23.0) | |||||
| Grade III | 26 (4.4) | 8 (2.3) | 5 (2.3) | 4 (1.9) | |||||
| Grade IV | 1 (0.2) | 1 (0.3) | 1 (0.5) | 1 (0.5) | |||||
| Unknown | 83 (13.9) | 60 (17.1) | 37 (17.4) | 38 (17.8) | |||||
| Radiation, n (%) | <0.001 | 0.237 | 0.34 | 0.124 | |||||
| No/unknown | 593 (99.5) | 337 (96.0) | 210 (98.6) | 206 (96.7) | |||||
| Yes | 3 (0.5) | 14 (4.0) | 3 (1.4) | 7 (3.3) | |||||
| Chemotherapy, n (%) | 0.60 | 0.048 | >0.99 | 0.030 | |||||
| No/unknown | 579 (97.1) | 338 (96.3) | 208 (97.7) | 207 (97.2) | |||||
| Yes | 17 (2.9) | 13 (3.7) | 5 (2.3) | 6 (2.8) | |||||
| Lymphadenectomy, n (%) | <0.001 | 1.070 | >0.99 | 0.015 | |||||
| No | 24 (4.0) | 156 (44.4) | 24 (11.3) | 23 (10.8) | |||||
| Yes | 572 (96.0) | 195 (55.6) | 189 (88.7) | 190 (89.2) | |||||
| No. malignancy, mean (SD) | 1.66 (0.93) | 1.97 (1.10) | <0.001 | 0.301 | 1.86 (1.06) | 2.01 (1.15) | 0.18 | 0.132 | |
LIMA, lung invasive mucinous adenocarcinoma; SEER, Surveillance, Epidemiology, and End Results; PSM, propensity score matching; SMD, standardized mean difference.
Survival comparison
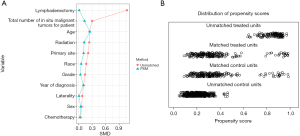
The long-term outcomes of LIMA patients were analyzed (Figure 3). Pre-PSM data indicated that patients undergoing lobar resection demonstrated superior survival rates compared to those receiving sublobar resection, both in terms of OS (P=0.007) and CSS (P=0.03). Post-PSM, however, no discernible differences in OS and CSS were observed (P=0.44 and P=0.23, respectively). Further validation was sought through survival analysis using IPTW, which also confirmed the absence of significant differences in OS and CSS post-PSM (P=0.71 and P=0.26, respectively).
Cox regression analysis
Both univariate and multivariate Cox regression analyses were undertaken to identify risk determinants for OS and CSS. For the entire cohort, preliminary findings underscored factors such as age, gender, differentiation grade, radiotherapy, chemotherapy, lymphadenectomy, resection extent, and No. malignancies as pivotal indicators influencing OS. On subjecting these determinants to a multivariate analysis, age, gender, differentiation grade, chemotherapy, lymphadenectomy, and No. malignancies emerged as unequivocal predictors of OS in LIMA patients. In contrast, radiotherapy and resection extent did not exhibit statistical significance (refer to Table S1). The Cox regression analysis pertaining to CSS is detailed in Table S2, while findings from the PSM and IPTW cohorts are documented in Tables S3-S6, all of which are congruent.
Effect of resection extent on long-term survival
Table 2 encapsulates the impact of surgical procedure (sublobar resection vs. lobar resection) on prolonged survival. Prior to PSM, the univariate analysis indicated a heightened risk associated with sublobar resection (HR =1.371, 95% CI: 1.091–1.724, P=0.007 for OS and HR =1.425, 95% CI: 1.043–1.949, P=0.03 for CSS) relative to lobar resection. Yet, the multivariate analysis portrayed a comparable risk between the two. Post-PSM and IPTW, the risk associated with sublobar resection mirrored that of lobar resection.
Table 2
| Survival | Cohort | Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| OS | Primary | 1.371 | 1.091–1.724 | 0.007 | 1.102 | 0.835–1.454 | 0.49 | |
| PSM | 1.144 | 0.813–1.609 | 0.44 | 1.141 | 0.803–1.62 | 0.46 | ||
| IPTW | 0.938 | 0.65–1.353 | 0.73 | 1.087 | 0.818–1.446 | 0.57 | ||
| CSS | Primary | 1.425 | 1.043–1.949 | 0.03 | 1.206 | 0.826–1.762 | 0.33 | |
| PSM | 1.359 | 0.822–2.248 | 0.23 | 1.305 | 0.778–2.188 | 0.31 | ||
| IPTW | 1.358 | 0.89–2.07 | 0.16 | 1.439 | 0.966–2.143 | 0.07 | ||
*, adjusted by the variables of age, sex, grade, radiation, chemotherapy, lymphadenectomy, No. malignancy. OS, overall survival; CSS, cancer-specific survival; LIMA, lung invasive mucinous adenocarcinoma; PSM, propensity score matching; IPTW, inverse probability of treatment weight; HR, hazard ratio; CI, confidence interval.
Subgroup analysis
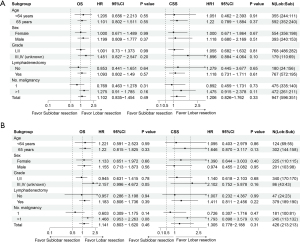
A supplementary subgroup analysis was executed to corroborate the results, juxtaposing sublobar resection and lobar resection, as visualized in Figure 4. Neither OS nor CSS displayed significant HRs across all subgroups (all P>0.05).
Discussion
Due to the infrequent occurrence of LIMA, no prior study has delved into the comparative effectiveness of resection extent. In the present study comparing resection extent for LIMA, we found that: (I) initial disparities in survival outcomes between lobar resection and sublobar resection were observed; (II) however, after careful patient selection and PSM, the resection extent did not significantly impact long-term survival; (III) key predictors of survival in LIMA patients included age, gender, differentiation grade, chemotherapy, lymphadenectomy, and No. malignancies; (IV) these findings align with recent research challenging the dominance of lobar resection in lung cancer surgery, suggesting that sublobar resection may be a viable option for select LIMA patients.
LIMA represents a unique histological subtype within the spectrum of adenocarcinomas, accounting for 2% to 5% of all adenocarcinoma patients (14). Owing to its rarity, there is a scarcity of survival data available for LIMA, often marked by contradictory findings. Notably, KRAS mutations are notably more prevalent in LIMA, while EGFR mutations are relatively infrequent, setting it apart from more common adenocarcinoma variants (15). Significantly, NRG1 gene fusions are emerging as crucial therapeutic targets for LIMA, suggesting that Afatinib could hold promise as an effective treatment option (16). Nevertheless, the prognosis of LIMA remains a subject of ongoing debate (17). Matsui et al. revealed notable differences between the LIMA group and the non-LIMA group (6). Specifically, the LIMA group exhibited a lower occurrence of lymph node metastasis (3% vs. 35%; P<0.01), a higher prevalence of KRAS mutations (56% vs. 9%; P<0.01), and a greater rate of intrapulmonary recurrence (84% vs. 31%; P<0.01) in comparison to the non-LIMA group. Interestingly, the 5-year OS rates did not exhibit a significant difference between the two groups (74% vs. 81%; P=0.26). However, it is worth noting that among patients who experienced intrapulmonary recurrence, individuals in the LIMA group faced a significantly worse prognosis than those in the non-LIMA group (35% vs. 77%; P<0.01) (6). Beck et al. found that LIMA that exhibits spontaneous regression of airspace opacities (SRAs) on serial CT scans tend to manifest as larger and multifocal lesions and typically display a pneumonic appearance. Additionally, patients diagnosed with LIMA showing SRAs typically present at a more advanced stage of the disease, which is associated with a higher mortality rate and reduced OS (18). Given LIMA’s propensity for a higher intrapulmonary relapse rate and a decreased likelihood of lymph node metastasis, lobar resection appears to be more favorable than sublobar resection. Nevertheless, there is very limited evidence of the surgical efficacy for LIMA, especially investigation of the resection extent.
More recent research, such as the JCOG0802 trial, has ascertained the equivalent efficacy of sublobar resection for early-stage NSCLC, challenging the long-standing dominance of lobar resection. Additionally, the CALGB 140503 trial demonstrated that sublobar resection was non-inferior to lobar resection in certain NSCLC patients (peripheral NSCLC with a tumor size of 2 cm or less and pathologically confirmed node-negative disease in the hilar and mediastinal lymph nodes). In the JCOG0802 and CALGB 140503 trials, surgical effectiveness in LIMA patients wasn’t delineated, as the histology subgroup analysis only categorized adenocarcinoma, squamous cell carcinoma, and other types. In addition, sublobar resection showed inferiority under some circumstances. Baig et al. demonstrated that for small peripheral NSCLC tumors measuring ≤2 cm and characterized by high tumor differentiation grades, the evidence suggests that lobar resection is linked to superior long-term survival outcomes compared to segmentectomy. They suggested that it’s important to stratify different NSCLC histologies in conjunction with their respective grades and this finer stratification would enable more precise selection criteria for segmentectomy as a treatment approach (19).
In this retrospective analysis, the choice between sublobar and lobar resections for patients with stage IA LIMA was influenced by several critical factors, primarily tumor characteristics such as size and location, as well as patient-specific considerations including overall pulmonary function and existing comorbidities. The decision to opt for sublobar resection, which typically includes procedures such as segmentectomy or wedge resection, was often guided by the intent to maximize lung tissue preservation while ensuring complete tumor removal. This approach is particularly favored in scenarios where tumors are smaller or located peripherally, allowing for effective disease management with minimal impact on lung capacity. It is important to note that the definitive histological confirmation of LIMA in many cases was only obtained postoperatively. This retrospective nature of the study highlights the clinical context and decision-making process at the time of surgery, reflecting real-world practices where surgical strategies are frequently determined by immediate clinical assessments and the need to balance oncologic control with quality-of-life considerations.
In the supplementary subgroup analysis depicted in Figure 4, we assessed the differential impacts of sublobar versus lobar resection on OS and CSS across various patient subgroups. The analysis was designed to explore whether certain characteristics influenced the effectiveness of the surgical approaches. The results indicated that there were no statistically significant differences in HRs for OS or CSS across all examined subgroups, with all P values exceeding 0.05. This uniformity across subgroups suggests that the relative efficacy of sublobar resection compared to lobar resection is consistent regardless of patient demographic or tumor-specific variables. These findings support the notion that sublobar resection can be considered a viable alternative to lobar resection for stage IA LIMA patients, without substantial variations in survival outcomes across different patient profiles. However, the lack of significant subgroup effects also prompts further investigation into other factors that might influence surgical outcomes, such as surgical technique specifics, post-operative care, and longer-term follow-up studies. Our results also demonstrated that differentiation grade was the independent risk factor for the long-term outcomes of LIMA patients. What’s more, in the subgroup analysis with high grade (III–IV), patients receiving sublobar resection are subjected to higher risk in OS compared with lobar resection, but without statistical significance (HR =2.157, 95% CI: 0.996–4.672, P=0.051). Additional research with a prospective design and a larger sample size is needed to determine if lobar resection is more beneficial for patients with high-grade LIMA.
Our analysis included 947 LIMA patients from the SEER database, spanning the years 2004 to 2016. Initially, baseline characteristics revealed variations between the lobar resection and sublobar resection groups, with age, tumor location, and No. malignancies influencing the choice of surgery. However, after PSM, the two groups demonstrated comparable baseline profiles, allowing for a more equitable comparison. Before PSM, our findings suggested that lobar resection was associated with significantly better OS and CSS than sublobar resection. Yet, these disparities diminished post-PSM, emphasizing the importance of careful patient selection in interpreting surgical outcomes. To further validate our results, we conducted survival analysis using IPTW, which affirmed the lack of significant differences in OS and CSS between lobar resection and sublobar resection after adjusting for potential confounders.
Zhang et al. conducted the multivariate Cox regression analysis in LIMA, and they found that the extent of surgery was able to predict the OS of LIMA patients. Using sublobar resection as reference, both lobar resection (HR =0.579, 95% CI: 0.434–0.773, P<0.0001) and pneumonectomy (HR =0.453, 95% CI: 0.259–0.791, P=0.005) demonstrated lower risk (8). However, Our Cox regression analysis showed that sublobar resection and lobar resection had similar risk in OS and CSS, which means that the choice of resection extent does not significantly impact survival outcomes in the multivariate analysis, echoing the post-PSM findings. The difference between Zhang’s study and our findings could be attributed the different TNM stage. Zhang et al. included all TNM stage patients, while we only focused on stage pT1N0M0. Identified key determinants of survival in LIMA patients include age, gender, differentiation grade, chemotherapy, lymphadenectomy, and No. malignancies, which were in accordance with previous studies (8).
Several limitations must be noted in this study. First, The SEER database lacks comprehensive information, including smoking history, surgical approach, and comorbidities, which might affect the multivariate analysis results. Second, Information about local recurrence in SEER database is not available, which is important regarding the surgical extent. Third, retrospective studies inherently have bias due to uneven distribution of prognostic factors between groups exposed and not exposed to the intervention. Last, our study is limited by the SEER database’s lack of detailed radiographic data, such as solid size and other CT characteristics. These elements are crucial for evaluating surgical outcomes and planning. The absence of this data restricts our ability to fully understand how tumor characteristics influence surgical decisions and patient prognoses. Future research on LIMA should explore randomized controlled trials that evaluate the impact of genetic markers on surgical outcomes. Integrating these findings with emerging therapies could revolutionize treatment strategies, enhancing efficacy and patient safety. Such studies are crucial for developing personalized surgical approaches that improve survival rates and quality of life for LIMA patients.
Conclusions
In conclusion, sublobar resection provides comparable efficacy with lobar resection in long-term outcomes of stage IA LIMA patients. If we determine that the histology is LIMA, there’s no necessity to widen the resection scope or opt for lobar resection. Further research, including prospective studies and randomized trials, is needed to refine these findings and guide clinical practice for LIMA patients.
Acknowledgments
During the preparation of this work the authors used ChatGPT4.0 in order to polishing the language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-24-3/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-24-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-24-3/coif). W.F. serves as an unpaid Executive Editor-in-Chief of Shanghai Chest. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Buettner R. Invasive mucinous adenocarcinoma: genetic insights into a lung cancer entity with distinct clinical behavior and genomic features. Mod Pathol 2022;35:138-9. [Crossref] [PubMed]
- Xie GD, Liu YR, Jiang YZ, et al. Epidemiology and survival outcomes of mucinous adenocarcinomas: A SEER population-based study. Sci Rep 2018;8:6117. [Crossref] [PubMed]
- Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res 2017;6:508-12. [Crossref] [PubMed]
- Lee HY, Cha MJ, Lee KS, et al. Prognosis in Resected Invasive Mucinous Adenocarcinomas of the Lung: Related Factors and Comparison with Resected Nonmucinous Adenocarcinomas. J Thorac Oncol 2016;11:1064-73. [Crossref] [PubMed]
- Matsui T, Sakakura N, Koyama S, et al. Comparison of Surgical Outcomes Between Invasive Mucinous and Non-Mucinous Lung Adenocarcinoma. Ann Thorac Surg 2021;112:1118-26. [Crossref] [PubMed]
- Kim DH, Bae SY, Na KJ, et al. Radiological and clinical features of screening-detected pulmonary invasive mucinous adenocarcinoma. Interact Cardiovasc Thorac Surg 2022;34:229-35. [Crossref] [PubMed]
- Zhang G, Wang X, Jia J, et al. Development and validation of a nomogram for predicting survival in patients with surgically resected lung invasive mucinous adenocarcinoma. Transl Lung Cancer Res 2021;10:4445-58. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Kagimoto A, Tsutani Y, Kushitani K, et al. Segmentectomy vs Lobectomy for Clinical Stage IA Lung Adenocarcinoma With Spread Through Air Spaces. Ann Thorac Surg 2021;112:935-43. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Chang WC, Zhang YZ, Nicholson AG. Pulmonary invasive mucinous adenocarcinoma. Histopathology 2024;84:18-31. [Crossref] [PubMed]
- Hwang DH, Sholl LM, Rojas-Rudilla V, et al. KRAS and NKX2-1 Mutations in Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 2016;11:496-503. [Crossref] [PubMed]
- Guo M, Tomoshige K, Meister M, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med 2017;9:462-81. [Crossref] [PubMed]
- Xu L, Li C, Lu H. Invasive mucinous adenocarcinoma of the lung. Transl Cancer Res 2019;8:2924-32. [Crossref] [PubMed]
- Beck KS, Sung YE, Lee KY, et al. Invasive mucinous adenocarcinoma of the lung: Serial CT findings, clinical features, and treatment and survival outcomes. Thorac Cancer 2020;11:3463-72. [Crossref] [PubMed]
- Baig MZ, Razi SS, Weber JF, et al. Lobectomy is superior to segmentectomy for peripheral high grade non-small cell lung cancer ≤2 cm. J Thorac Dis 2020;12:5925-33. [Crossref] [PubMed]
Cite this article as: Wang Q, Lv Y, Li K, Liu G, Fang W. Comparative efficacy of lobar resection and sublobar resection in patients with stage IA lung invasive mucinous adenocarcinoma: insights from the SEER database with propensity score matching. Shanghai Chest 2024;8:13.


