
A silent anomaly: congenital unilateral absence of pulmonary artery incidentally discovered in adulthood, presenting as pulmonary hypertension and chronic respiratory failure—case report
Highlight box
Key findings
• Unilateral absence of the pulmonary artery (UAPA) is a rare congenital anomaly usually identified during adolescence. However, atypical presentations prompted by the development of pulmonary hypertension (PH) and respiratory failure may lead to diagnosis in mid-adulthood with worsening clinical condition.
What is known and what is new?
• Due to slow progression of the disease, individuals with UAPA frequently experience symptoms for a prolonged time without fully grasping the gravity of their situation, ultimately resulting in adverse consequences.
• Managing UAPA poses challenges due to the absence of a universally accepted treatment protocol. Treatment is typically tailored to the individual’s clinical presentation. In cases of PH, pharmacotherapy with vasodilators is commonly pursued, whereas surgical options like selective embolization are reserved for individuals experiencing massive hemoptysis. Notably, lung transplant may emerge as a definitive treatment option.
What is the implication, and what should change now?
• The gradual advancement of UAPA and its atypical presentations lead to patients experiencing symptoms for an extended duration without recognizing the gravity of their condition. Clinicians must maintain a high index of suspicion and conduct thorough scrutiny, employing both general and specialized investigations, when determining the underlying cause.
Introduction
Unilateral absence of the pulmonary artery (UAPA) is a rare congenital anomaly resulting from the failure of the sixth aortic arch to connect with the pulmonary trunk during embryogenesis (1,2). Left-sided UAPA is more commonly associated with congenital cardiovascular malformations making it easy to diagnose and treat early in life, whereas right-sided UAPA is often asymptomatic and incidentally discovered on chest radiographs (3,4). Although UAPA is typically diagnosed in adolescence (average age: 14 years), we encountered atypical presentations at our institute leading to diagnoses in mid-adulthood, prompted by symptoms of pulmonary hypertension (PH) and respiratory failure.
The scarcity of literature regarding isolated UAPA presents challenges in diagnostic and therapeutic planning (5). Therefore, our goal is to add to the existing knowledge base, develop a deeper understanding of its clinical presentation, management, and the ability to predict outcomes. The cases presented originate from a single-center, retrospective design and are non-consecutive. We present this article in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-48/rc).
Case presentation
Case 1
A 44-year-old man was brought to the emergency department with hypoxia, hemoptysis, and progressive dyspnea. He complained of nonproductive cough, intermittent wheezing, and progressive dyspnea with minimal activity over the previous year. He was a heavy smoker and diagnosed with chronic obstructive pulmonary disease (COPD), requiring ongoing home oxygen treatment. His medical history was noteworthy for poorly controlled COPD exacerbations attributed to noncompliance with prescribed medications, leading to recurrent emergency room visits. Further investigation revealed an extensive history of substance abuse and nonadherence to oxygen therapy. Physical examination revealed mild lower extremity edema and bilateral expiratory wheezing. The patient was initially stabilized on fluids, his hemoptysis resolved spontaneously without any further episodes or need for embolization. Subsequent imaging, with a contrast-enhanced computed tomography (CT), did not reveal any active bleeding sites. On further workup, chest X-ray demonstrated a small-volume right hemithorax with increased reticular opacities, mild rightward shift of the cardiomediastinal silhouette, an empty right pulmonary bay (hilum), and compensatory hypertrophy of the left lung (Figure 1). These findings were further characterized on contrast-enhanced CT, revealing a markedly dilated main pulmonary arterial trunk measuring up to 4.2 cm in transverse diameter consistent with severe pulmonary arterial hypertension (PAH), an absent right main pulmonary artery (PA), and extensive systemic arterial collateralization in the hypoplastic right lung, most compatible with congenital proximal interruption of the PA. Moderate emphysema and compensatory hyperinflation of the left lung was also observed (Figure 2). Electrocardiogram showed a normal sinus rhythm with right axis deviation, incomplete right bundle branch block, and right ventricular hypertrophy. A limited echocardiogram study revealed a dilated right ventricle with reduced function. Once stable, the patient underwent a right heart catheterization, revealing a mean PA pressure of 34 mmHg, a right atrial (RA) pressure of 7 mmHg, and a PA wedge pressure of 10 mmHg. The patient was continued on bronchodilators and supplemental oxygen therapy. Sildenafil and ambrisentan for PAH were initiated and spironolactone 20 mg, 3 times a day, was administered to help with his volume status. Despite initial stabilization, the patient succumbed to ongoing right ventricular failure and dysrhythmias leading to cardiac arrest.
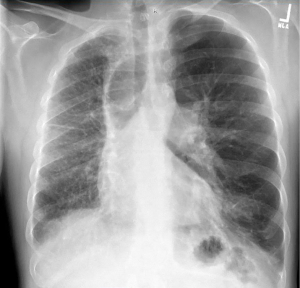

Interestingly, a CT angiogram done a year previously revealed absence of the right PA, with perfusion to the right lung being supplemented through collateral vessels originating from the bronchial artery, the costal artery, or the thoracic aorta. The reduced volume of the right lung suggested the possibility of a congenitally small lung; however, the cardiac contour was normal without evidence of congenital cardiac defects. Additionally, hypertrophy of the left PA was observed, along with clear visualization of the aorta and the left PA, including its segmental branches. At that time, the patient was referred to a lung transplant center but was lost to follow-up.
Case 2
A 46-year-old woman of Ghanaian descent with a history of uncontrolled asthma presented to the emergency department with symptoms of asthma exacerbation that had been present for 2 days. Since being diagnosed with asthma at the age of 31, she has had regular visits to the emergency department for upper respiratory infections. She also reported a considerable history of dyspnea with exertion, particularly during daily activities, and previous respiratory distress due to an allergic response to aspirin. Furthermore, her family history revealed several incidences of nasal polyps among her relatives.
A chest X-ray revealed reduced lung volume on the right side as well as ipsilateral heart and mediastinum displacement. Chest CT and chest X-rays revealed absence of the right PA (Figures 3,4), resulting in a hypoplastic right lung fed by a hypertrophied bronchial artery branching out from the aorta. Furthermore, the underdeveloped right lung revealed chronic interstitial changes, scarring, and bronchiectasis. A ventilation-perfusion scan revealed a lack of blood flow to the right lung as well as perfusion abnormalities in the base of both lung (Figures 4,5). Echocardiogram revealed a PA pressure of 77 mmHg with a right ventricular systolic pressure of 76.7 mmHg. In addition, moderate tricuspid regurgitation and mild right ventricular dilatation along with moderate right ventricular hypokinesis was noted.



The patient was admitted to the hospital and treated with oral corticosteroids and a nebulizer and exhibited remarkable clinical improvement within 3 days. An outpatient right heart catheterization to evaluate the possibility of PH revealed an elevated mean RA pressure of 10 mmHg, a right ventricular pressure of 45/10 mmHg, and an end-diastolic pressure of 12 mmHg. The left PA pressure was 46/24/30 mmHg, and the pulmonary capillary wedge pressure was 10 mmHg, with an approximate transpulmonary gradient of 20 mmHg. Cardiac output, calculated using the FICK equation, was 4.56 L per minute, with a cardiac index of 3.55 L per minute/m2. The calculated pulmonary vascular resistance was 4.38 mmHg × min/L or 350 per cm3.
Oxygen saturation measurements demonstrated a rise in saturation from the superior vena cava to the right atrium, indicating the presence of a left-to-right shunt attributed to collateral circulation. The partial pressure of oxygen in the aorta climbed to 484 mmHg after a nitric oxide challenge (20 ppm) and continued high-flow oxygenation, while the left PA pressure increased to 52 mmHg. After a 5-minute period, the aortic partial pressure increased to 514 mmHg, indicating a good response to the vasodilatory effects of nitric oxide.
An aortogram revealed a large trifurcating fistulous connection originating from the proximal descending aorta and extending to the underdeveloped right lung, with indications of sequestration observed as contrast material entered the heart chambers during the contrast injection phase. Consequently, the patient underwent a complex structural intervention, involving the placement of seven coils: two in the superior, three in the middle, and two in the inferior collateral vessels, resulting in an excellent technical outcome.
Ethical consideration
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
UAPA is an extremely rare congenital malformation that affects 1 in 200,000 adults. In most cases, the right PA, opposite to the aortic arch, is affected due to altered development of the sixth aortic arch segment that fails to connect with the pulmonary trunk (4). Subsequently, extensive systemic collateral branches develop to supply the ipsilateral peripheral pulmonary arteries, which can often lead to hemoptysis, as seen in the patient presented in case 1. UAPA rarely occurs in isolation, as it did in this case. It is more frequently seen in conjunction with congenital cardiac defects such as tetralogy of Fallot or septal defects (1-3).
Dyspnea, exercise intolerance, recurrent chest infections, PH, hemoptysis, and chest pain are all nonspecific clinical manifestations of UAPA. Therefore, a thorough evaluation is necessary to diagnose this condition as it is easy to miss. A chest radiograph will classically show ipsilateral volume loss with diaphragmatic elevation and shift of the mediastinum toward the affected side as well as hyperaeration of the contralateral lung (2,3,5). Fine non-branching linear opacities can be seen at the lung periphery indicating enlarged intercostal and transpleural pulmonary collaterals. Pulmonary angiography is the gold standard for diagnosing UAPA, however, CT, magnetic resonance imaging (MRI), and echocardiogram are more often used to confirm the diagnosis (6).
Isolated UAPA with PH has rarely been documented, and risk factors for the development of PH are unknown. In most cases, PH is mild, unlike our patient in case 1 for which right heart catheterization findings confirmed the diagnosis of severe PH. According to one theory, PH develops as a result of pulmonary arterial vascular remodeling, which occurs in response to increased blood flow through the single patent PA leading to endothelial intimal injury, intimal hyperplasia, medial hypertrophy, and ultimately collagenous replacement of the intima in advanced disease. Although most cases of PH have been described as mild, PH in the patient described in case 1 may have been exacerbated by his past noncompliance with O2 therapy, multiple COPD exacerbations, and substance abuse in addition to his UAPA (6,7).
Since a universally accepted form of therapy does not exist, UAPA continues to pose therapeutic challenges. Serial echocardiography is used to monitor asymptomatic patients for the emergence of PH. Adult patients receive therapy that is specific to their clinical presentation. Due to extensive fibrosis of the intrapulmonary arteries, surgical options are often not viable (8). Therefore, patients with PH have largely pursued pharmacotherapy with vasodilators like endothelin receptor antagonists, calcium channel blockers, and intravenous prostacyclins with improvements seen in their dyspnea and PH. Massive hemoptysis may be treated with selective embolization of the systemic collateral circulation, and pneumonectomy may be preferred in those with recurrent hemoptysis, with the exception of high risk patients due to the technical difficulties with embolization brought on by the extensive collateral circulation present in UAPA (8-10). Despite a favorable response to medical therapy observed in our patient in case 2, it was deemed necessary for her to undergo a complex structural intervention to attain the optimal outcome. Conversely, our patient in case 1 exhibited resistance to all medical interventions, leading to the determination that lung transplantation remained the most suitable course of treatment.
Conclusions
To summarize, given the slow progression of their disease, patients with UAPA often experience symptoms for an extended period without recognizing the seriousness of their condition which eventually leads to detrimental effects. This case highlights the diagnostic and therapeutic challenges that patients with rare causes of PH may experience, as well as the need for a high index of suspicion and meticulous scrutiny of both general and specialized investigations when establishing the etiology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-48/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khaladkar SM, Kumar D, Kapoor R. Isolated unilateral absence of pulmonary artery- an unusual cause of hemoptysis: a case report. The Egyptian Journal of Bronchology 2023;17:10. [Crossref]
- Reading DW, Oza U. Unilateral absence of a pulmonary artery: a rare disorder with variable presentation. Proc (Bayl Univ Med Cent) 2012;25:115-8. [Crossref] [PubMed]
- Presbitero P, Bull C, Haworth SG, et al. Absent or occult pulmonary artery. Br Heart J 1984;52:178-85. [Crossref] [PubMed]
- Wang P, Yuan L, Shi J, et al. Isolated unilateral absence of pulmonary artery in adulthood: a clinical analysis of 65 cases from a case series and systematic review. J Thorac Dis 2017;9:4988-96. [Crossref] [PubMed]
- Aypak C, Yıkılkan H, Uysal Z, et al. Unilateral absence of the pulmonary artery incidentally found in adulthood. Case Rep Med 2012;2012:942074. [Crossref] [PubMed]
- Kruzliak P, Syamasundar RP, Novak M, et al. Unilateral absence of pulmonary artery: pathophysiology, symptoms, diagnosis and current treatment. Arch Cardiovasc Dis 2013;106:448-54. [Crossref] [PubMed]
- Shostak E, Sarwar A A. 50-year-old woman with dyspnea, lower extremity edema, and volume loss of the right hemithorax. Chest 2009;136:628-32. [Crossref] [PubMed]
- Ten Harkel AD, Blom NA, Ottenkamp J. Isolated unilateral absence of a pulmonary artery: a case report and review of the literature. Chest 2002;122:1471-7. [Crossref] [PubMed]
- Reñé M, Sans J, Dominguez J, et al. Unilateral pulmonary artery agenesis presenting with hemoptysis: treatment by embolization of systemic collaterals. Cardiovasc Intervent Radiol 1995;18:251-4. [Crossref] [PubMed]
- Turner D, Vincent J, Epstein M. Isolated right pulmonary artery discontinuity. Images Paediatr Cardiol 2000;2:24-30. [PubMed]
Cite this article as: Moin A, Trahan A, Mody M, Mohamed H, Arjuna A. A silent anomaly: congenital unilateral absence of pulmonary artery incidentally discovered in adulthood, presenting as pulmonary hypertension and chronic respiratory failure—case report. Shanghai Chest 2024;8:11.


