
Lungs under siege: exploring a rare case of diffuse alveolar-septal pulmonary amyloidosis—a case report
Highlight box
Key findings
• Diffuse alveolar and septal amyloidosis represents an exceptionally uncommon presentation of pulmonary amyloidosis, characterized by limited known treatment strategies and an overall survival rate of approximately 13 months.
What is known and what is new?
• Distinguishing diffuse alveolar septal amyloidosis from other interstitial and granulomatous diseases is highly challenging due to their similar symptoms and imaging findings. Consequently, the condition is frequently prone to misdiagnosis.
• Bronchoscopy and transbronchial lung biopsy are deemed relatively safe approaches for antemortem diagnosis of this condition when conducted with caution.
What is the implication, and what should change now?
• Given the absence of established standard treatments for this disease, current therapeutic approaches primarily involve chemotherapy and steroids as the primary treatment. Nevertheless, it is advisable to regard lung transplant as a viable treatment option for such cases.
Introduction
Amyloidosis is a rare multisystem disease with an estimated incidence of 6 to 10 cases per 100,000 annually in Western Europe and the United States (1,2). It is characterized by deposition of insoluble misfolded proteins in different tissues and organs throughout the body. Pulmonary involvement in amyloidosis occurs in only half of the cases, with five types recognized on imaging (Table 1). Among these, the diffuse alveolar septal subtype is the rarest, usually diagnosed on autopsy, and is often associated with plasma cell dyscrasias and an extremely poor prognosis (3).
Table 1
| Classification | Imaging |
|---|---|
| Alveolar septal | Interlobular septal thickening with traction bronchiectasis |
| Nodular | Round nodules with sharp margins |
| Adenopathy | Extrathoracic nodes most common. Often calcified with eggshell or popcorn appearance |
| Pleural | Pleural studding |
| Tracheo-bronchial | Tracheal and bronchial wall thickening. Presents with post-obstructive pneumonia or atelectasis |
AL, amyloid light chain or primary amyloidosis.
We present an exceptionally rare pattern of systemic primary or light chain (AL) amyloidosis with diffuse alveolar septal involvement in an elderly woman prompting further literature review and scientific reporting to improve our understanding of this unique disease process (4). We present this article in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-49/rc).
Case presentation
A 72-year-old woman with a 10-pack-year history of smoking and systemic amyloidosis presented to our department with complaints of a persistent cough and progressively worsening shortness of breath over the past 3 months. Physical examination did not reveal any significant findings. Chest X-ray showed increased interstitial markings bilaterally and a calcified granuloma in the lower right lobe (Figure 1).
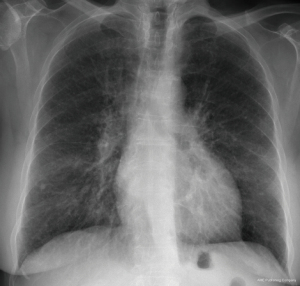
Blood tests, including complete blood count, C-reactive protein, renal function, and liver function returned within normal ranges. Cardiac evaluation through echocardiography revealed a slightly diminished ejection fraction alongside global left ventricular hypokinesis and evidence of left ventricular diastolic dysfunction. Further diagnostic insights were obtained via right heart catheterization with biopsy, which indicated a mean pulmonary artery (PA) pressure of approximately 32 mmHg (with systolic and diastolic pressures of 48 and 26 mmHg, respectively), alongside a pulmonary capillary wedge pressure of 22 mmHg. These findings suggested the presence of pulmonary hypertension, and the biopsy established a diagnosis of AL amyloidosis.
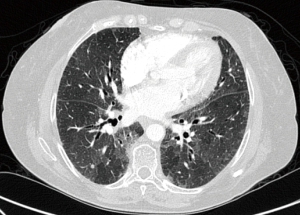
A high-resolution chest computed tomography (CT) revealed scattered, non-calcified, solid centrilobular nodules with mild, diffuse bronchiectasis along with bilateral hilar and mediastinal lymphadenopathy (Figure 2).
Fiberoptic bronchoscopy was performed with transbronchial biopsy of the right middle lobe, which revealed lesions consistent with diffuse alveolar-septal amyloidosis. The specimens showed deposition of amyloid material within the alveolar septa and surrounding tissues. While the lesions were generally hypocellular, scattered plasma cells were observed within the affected areas. Importantly, no giant cells were identified. Additionally, diffuse light chain deposits were found on biopsy, and immunofluorescence evaluation indicated diffuse alveolar septal involvement by immunoglobulin G (IgG) lambda (heavy/light chain), consistent with light chain lambda disease.
Congo red staining & AA (secondary amyloidosis) amyloid testing results were negative; however, amyloid P component and thioflavin T stain tested positive. Additionally, electron microscopy of the pulmonary parenchyma specimen revealed expanded alveolar septa and focal nodules containing amorphous material. Fibrillary material was randomly deposited in the alveolar septa, forming non-branching nodules that measured 7 to 12 nm in diameter and obliterated the normal pulmonary architecture. Bone marrow examination revealed 6% to 10% plasma cells with lambda light chain restriction, consistent with monoclonal gammopathy associated with amyloidosis. A serum light chain assay demonstrated 10.9 mg/L kappa free light chains with a ratio of 0.02. The M-spike was 0.23 g/dL IgG lambda, and beta-2-microglobulin was 2.72 mg/L, which confirmed the diagnosis of multiple myeloma, lambda light chain type after extensive review by the hematology and oncology specialists. The patient was enrolled in a clinical trial and received four courses of chemotherapy consisting of bortezomib and dexamethasone weekly with remarkable improvements in her respiratory symptoms. Repeat bone marrow biopsy again revealed 6% to 10% plasma cells with lambda restriction, consistent with monoclonal gammopathy associated with amyloidosis, but no advanced myeloma (Figure 3). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Discussion
Amyloidosis is characterized by the extracellular deposition of insoluble fibers that disrupt organ function. It is classified according to the type of protein that has been deposited. The two major kinds are AL and AA, with AL amyloidosis characterized by systemic involvement and AA amyloidosis potentially affecting one or more organs (1).
Pulmonary involvement in patients with systemic amyloidosis rarely dominates the clinical picture and is typically identified postmortem, particularly the diffuse parenchymal variant, which is rarer and has only been documented in a handful of cases (5-8). Initially asymptomatic, this condition gradually progresses due to the damaging effects of amyloid deposits on the alveolar membrane, eventually manifesting as cough and dyspnea and potentially leading to pulmonary hypertension and respiratory failure (6). However, in this unique case, we made an antemortem diagnosis through transbronchial biopsies. We believe that documenting this case is significant as it highlights the possibility of early detection and intervention in rare instances of pulmonary amyloidosis, thereby potentially enhancing patient outcomes. In patients with this variant, amyloid nodules typically manifest in diffuse patterns, with focal deposits being less common. Often located in the lower lobes, these nodules often exhibit sharp or lobulated contours, accompanied by calcifications, and are frequently situated centrally or irregularly within the nodule on CT images (8). In our case, CT revealed multiple calcified nodules and lymphadenopathy in the mediastinum and hilum, which prompted bronchoscopy with biopsy to ensure that no concurrent pulmonary conditions were overlooked and to precisely characterize the nature of these nodules. Initially, the possibility of nodular pulmonary amyloidosis was entertained due to these imaging characteristics. However, upon further evaluation, the observed nodules did not align with the typical findings associated with nodular pulmonary amyloidosis. After histopathological confirmation, which revealed inert, proteinaceous, homogeneous, eosinophilic material, and consultation with pathology specialists, we established a diagnosis of diffuse alveolar septal amyloidosis.
Tissue biopsy is considered the gold standard for diagnosing and classifying pulmonary amyloidosis, and the presence of apple-green birefringence under polarized light of Congo red-stained tissue is crucial for distinguishing it from other diseases (1). In our case, Congo red staining was negative, despite positive amyloid P and thioflavin T staining. This discrepancy indeed raises the possibility of a false-negative result for Congo red staining, or it may suggest the presence of amyloid-like fibrils that do not exhibit the typical Congo red birefringence under polarized light microscopy. Such a scenario might indicate the presence of amyloid-related proteins or structures that share biochemical and morphological characteristics with amyloids but do not fulfill all the criteria for classical amyloid deposition. To address this discrepancy and validate our findings, we sent the pathology samples to a comprehensive center for amyloidosis for confirmation.
The list of differential diagnoses for diffuse parenchymal pulmonary amyloidosis, including pneumonia, pneumoconiosis, interstitial lung disease, and lymphangitic carcinomatosis, is extensive. This condition has a poor prognosis, with median survival dropping from 13 months in untreated cases to just 4 months if heart failure develops (6). In our patient, differentiating between primary amyloidosis (AL type) and diffuse alveolar amyloidosis was essential. She presented predominantly with respiratory symptoms and localized pulmonary findings without systemic symptoms or multiorgan involvement typically associated with primary amyloidosis (AL type). Additionally, imaging, particularly CT, showed diffuse reticular or nodular opacities in the lung parenchyma, whereas histological examination confirmed amyloid deposits in the alveolar septa, conclusively confirming the diagnosis of diffuse alveolar amyloidosis.
Historically, the rarity of amyloidosis has made randomized controlled trials challenging, leading to treatment approaches based on multiple myeloma therapies. Treatment goals include clearing amyloid deposits, hindering fibril formation, and stabilizing precursor proteins to prevent organ damage, with chemotherapy and steroids as primary treatments. Initially, melphalan (an alkylating agent that disrupts DNA synthesis) and dexamethasone (MDex) were used. The introduction of bortezomib, a proteasome inhibitor successful in myeloma treatment, marked a shift. A phase III trial (NCT01277016, January 2011–February 2016) demonstrated a superior hematologic response and survival with bortezomib, melphalan, and dexamethasone (BMDex) compared to MDex alone, despite side effects including cytopenia, neuropathy, and heart failure. CyBorD, substituting cyclophosphamide for melphalan, became a preferred treatment for some time (9). Our patient received bortezomib and dexamethasone, chosen for its rapid effect and high response rate. Although medical treatment initially improved our patient’s symptoms, the progressive nature of pulmonary amyloidosis raised concerns about long-term management and the potential for disease progression despite medical therapy. Given this, lung transplantation was considered as a potential option for definitive treatment and long-term management, particularly if the patient’s symptoms were to worsen over time.
In recent years, lung transplantation has been documented as a viable treatment option for cases of isolated pulmonary amyloidosis. In one case involving AL amyloidosis, the development of severe symptoms and pulmonary hypertension necessitated lung transplantation (10). Another case involved a patient with pulmonary hypertension due to amyloidosis secondary to systemic lupus erythematosus and Sjögren’s syndrome, who underwent bilateral lung transplantation and remained stable even 7 years after transplantation (6). These cases suggest that lung transplantation can offer favorable long-term outcomes for select patients with pulmonary amyloidosis, whether isolated or combined with pulmonary hypertension.
Conclusions
Our case highlights diffuse alveolar septal amyloidosis as a rare and fatal disorder that is often misdiagnosed given its variable presentation and imaging patterns. Radiologists and physicians should consider amyloid in clinically perplexing, chronically ill patients, particularly those with plasma cell dyscrasias or chronic inflammation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-49/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-49/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohammadien HA, Morsi SN, Al Shahat MA. Systemic AL amyloidosis presenting with diffuse alveolar septal involvement and respiratory failure: a case report and review of the literature. The Egyptian Journal of Bronchology 2021;15:23. [Crossref]
- Urban BA, Fishman EK, Goldman SM, et al. CT evaluation of amyloidosis: spectrum of disease. Radiographics 1993;13:1295-308. [Crossref] [PubMed]
- Sato H, Ono A, Okada F, et al. A Case of Diffuse Alveolar Septal Amyloidosis Associated With Multiple Myeloma. J Thorac Imaging 2015;30:W73-5. [Crossref] [PubMed]
- Hui AN, Koss MN, Hochholzer L, et al. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med 1986;110:212-8. [PubMed]
- Berk JL, O'Regan A, Skinner M. Pulmonary and tracheobronchial amyloidosis. Semin Respir Crit Care Med 2002;23:155-65. [Crossref] [PubMed]
- Ellender CM, McLean C, Williams TJ, et al. Autoimmune disease leading to pulmonary AL amyloidosis and pulmonary hypertension. Respirol Case Rep 2015;3:78-81. [Crossref] [PubMed]
- Gandham AK, Gayathri AR, Sundararajan L. Pulmonary amyloidosis: A case series. Lung India 2019;36:229-32. [PubMed]
- Poletti V, Costabel U, Casoni GL, et al. Rare infiltrative lung diseases: a challenge for clinicians. Respiration 2004;71:431-43. [Crossref] [PubMed]
- Moy LN, Mirza M, Moskal B, et al. Pulmonary AL amyloidosis: A review and update on treatment options. Ann Med Surg (Lond) 2022;80:104060. [Crossref] [PubMed]
- Ware LB, Keith FM, Gordon RL, et al. Lung transplantation for pulmonary amyloidosis: a case report. J Heart Lung Transplant 1998;17:1129-32. [PubMed]
Cite this article as: Moin A, Mody M, Arjuna A. Lungs under siege: exploring a rare case of diffuse alveolar-septal pulmonary amyloidosis—a case report. Shanghai Chest 2024;8:12.


