
Video-assisted thoracoscopic surgery for patients with non-small cell lung cancer larger than 5 cm
Highlight box
Key findings
• Video-assisted thoracic surgery (VATS) could be considered as a safe and feasible choice for those well-selected patients with non-small cell lung cancer (NSCLC) larger than 5 cm.
What is known and what is new?
• The size of NSCLC larger than 5 cm, which is classified into T3 and T4 stage tumors, is considered as locally advanced diseases. Surgical resection plays a role in the treatment of those T3 and resectable T4 NSCLCs, though radical resection is complicated
• For patients with NSCLC larger than 5 cm
• VATS not only showed comparable progression-free survival and overall survival (OS) in total in accordance with thoracotomy, but significantly benefited patients receiving adjuvant chemotherapy with better OS outcomes
What is the implication, and what should change now?
• For patients with NSCLC larger than 5 cm, VATS procedure is a preferable option.
Introduction
Non-small cell lung cancer (NSCLC) accounts for almost 70% of lung cancers and contributes to the leading cause of cancer-related mortality around the world (1). According to the 8th tumor node metastasis (TNM) classification, lung cancer is classified into T3 or T4 when tumor diameter is large than 5 cm, and tumor size is a prognostic factor for NSCLC (2). For locally advanced NSCLC (LA-NSCLC), including those larger than 5 cm, effectiveness of treatment is not satisfactory (3,4). For LA-NSCLC, especially T3 and resectable T4 tumors, radical surgical resection is still considered as a recommended treatment (5). As an important part of minimally invasive surgery, video-assisted thoracic surgery (VATS) has been strongly recommended for the resection of early-stage NSCLC, owing to better perioperative results and less postoperative complications compared with open thoracotomy (6-8). Tumor size larger than 5 cm is considered as a locally advanced disease and the indication of VATS approach is in suspense for the current clinical decision (9). According to the 2nd european society for medical oncology (ESMO) consensus on lung cancer in 2013, tumor size larger than 5 cm was regarded as a factor that made minimally invasive surgery inaccessible (10). In recent years, VATS has been gradually adopted not only for early-stage but also for LA-NSCLC of complicated resections, especially performed by experienced surgeons and medical centers (10,11). But the safety and feasibility as well as oncological prognosis of VATS resection for those NSCLC patients with tumor size larger than 5 cm still remain unclear.
Therefore, we aimed to study the safety and feasibility of VATS approach possessed acceptable and if it had any survival benefit for patients with NSCLC larger than 5 cm compared with open thoracotomy. We present this article in accordance with the STROBE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-39/rc).
Methods
Inclusion and exclusion criteria
Patients with NSCLC larger than 5 cm in diameter receiving surgical resection at Shanghai Chest Hospital from January 2013 to December 2017 were retrospectively collected and enrolled in this study. The inclusion and exclusion criteria were plotted in Figure 1. Patients would be excluded if they had small cell lung cancer, metastatic lesions, undefined pathology, or multiple primary NSCLCs confirmed by final pathology. Multiple positive N2 lymph node stations and N3 lymph node stations confirmed by preoperative positron emission tomography/computed tomography (PET/CT), endobronchial ultrasound (EBUS) biopsy or mediastinoscopy biopsy were excluded. Those patients with unresectable T4 or stage IV NSCLCs diagnosed by preoperative examination or intraoperative operation as well as patients receiving induction therapy were also excluded.
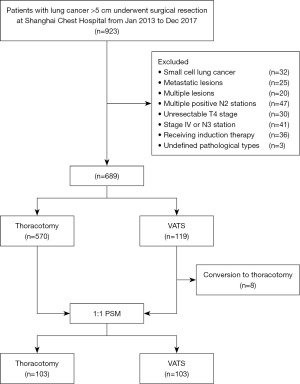
Patients who met the above inclusion and exclusion criteria were divided into two groups, the VATS group and the thoracotomy group. VATS group was defined as patients received complete VATS resection including those converted to open thoracotomy during operation according to the intention-to-treat analysis. Thoracotomy group was defined as patients receiving open thoracotomy for surgical resection. As for resection extent, lobectomy as well as other operations including bi-lobectomy, sleeve resection and pneumonectomy were all counted.
Research endpoints
The primary endpoint of this study was perioperative safety and feasibility. R0 resection is defined according to International Association for the Study of Lung Cancer (IASLC) recommendations. Intraoperative data including the average operation time, volume of blood loss, lymph node dissection (harvested lymph nodes, as well as positive lymph nodes), intraoperative complications were retrospectively counted from our lung cancer database. Postoperative data were as following, length of hospital stay (LOS) after surgery, chest drainage duration, rate of postoperative complications as well as rate of in-hospital mortality. Postoperative complications were described and counted according to the Common Terminology Criteria for Adverse Events Version (CTCAE) 5.0, including prolonged air leakage, arrhythmia, postoperative bleeding, bronchopleural fistula (BPF), chylothorax, pulmonary infection or pyothorax, pleural effusion, reoperation, readmission and others. Specifically, prolonged air leakage was defined as air leakage more than 5 days.
Secondary endpoints were cancer recurrence and survivals, including progression-free survival (PFS) and overall survival (OS) (1). PFS was calculated from the date of surgery to the date of cancer recurrence or patient death or last follow-up. OS was defined as the interval between the date of surgery and the date of patient death or last follow-up. For postoperative follow-up, patients were recommended to take routine postoperative examinations including serum tumor markers, chest CT and cervical and abdominal ultrasound every 3 months, as well as brain magnetic resonance imaging (MRI) and bone scan every 6 months during the first year and then repeated every year. PET/CT was required if necessary.
Statistical analysis
Continuous and categorical variables were presented as mean ± standard deviation (SD) and frequencies (percentages, %), respectively. Differences were tested by Student’s t-test for continuous variables and Pearson χ2 or Fisher’s exact test for categorical ones. Intraoperative and postoperative outcomes were compared between those two groups before and after propensity score matching (PSM) analysis. The VATS and thoracotomy groups were matched by gender, age, smoking history, body mass index (BMI), forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (DLCO), comorbidity, central location, tumor location, resection extent, tumor size, histology types, pathological T stage and pathological N stage. PSM was carried out with a Caliper value set at 0.1 of the standard deviation. Intraoperative and postoperative outcomes were compared between those two groups before and after PSM analysis. Survival curves were plotted by Kaplan-Meier analysis and verified by Log-rank test. Univariable and multivariable cox proportional hazard regressions were analyzed after PSM to identify independently risk factors for those patients with resected NSCLC large than 5 cm. In addition, subgroup analysis was also conducted according to patients receiving adjuvant chemotherapy (ACT) or not as well as those VATS patients who suffered conversion to open thoracotomy. Statistical difference was set as P<0.05 with two sides. All analyses were performed by SPSS (version 20.0), R (version 3.2.2) and GraphPad (Prism 5).
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Chest Hospital (No: IS21113) and individual consent for this retrospective analysis was waived.
Results
Clinicopathological characteristics of patients before PSM
A total of 689 patients with resected NSCLC large than 5 cm were enrolled. Among them 119 (17.3%) patients received VATS resection and 570 (82.7%) patients received thoracotomy. VATS was increasingly used over time, from 10.6% (18/170) in 2013 to 32.1% (43/134) in 2017 (Figure S1). In the VATS group, eight cases were converted to open thoracotomy, with a conversion rate of 6.7% (8/119). Clinical and pathological characteristics of patients before and after PSM are listed in Table 1. We found out that the VATS group contained relatively older patients with relatively better FEV1%, less central location, less squamous cell carcinoma (SCC), more pN0 stage and more lobectomy were found in the VATS group. After PSM, 103 VATS patients were well matched with 103 thoracotomy patients (Table 1). Rate of R0 resection, grade of tumor differentiation, pleural invasion (PI), lymphovascular invasion, and rate of ACT and radiotherapy were similar between VATS and open groups before and after PSM.
Table 1
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Thoracotomy (n=570) | VATS (n=119) | P | Thoracotomy (n=103) | VATS (n=103) | P | ||
| Gender | 0.499 | 0.133 | |||||
| Male | 479 (84.0) | 97 (81.5) | 90 (87.4) | 82 (79.6) | |||
| Female | 91 (16.0) | 22 (18.5) | 13 (12.6) | 21 (20.4) | |||
| Age (years) | 61.5±8.8 | 64.3±9.2 | 0.002 | 63.4±8.0 | 63.6±9.2 | 0.865 | |
| Smoking history | 0.928 | 0.640 | |||||
| No | 419 (73.5) | 87 (73.1) | 73 (70.9) | 76 (73.8) | |||
| Yes | 151 (26.5) | 32 (26.9) | 30 (29.1) | 27 (26.2) | |||
| BMI (kg/m2) | 22.8±3.2 | 23.4±3.4 | 0.060 | 23.0±3.3 | 23.1±2.8 | 0.813 | |
| FEV1 (%) | 82.2±14.8 | 93.6±69.7 | <0.001 | 86.2±15.4 | 86.9±17.4 | 0.734 | |
| DLCO (%) | 83.9±19.0 | 83.8±18.7 | 0.938 | 83.5±19.5 | 84.6±18.8 | 0.680 | |
| Comorbidity | 0.175 | 0.774 | |||||
| No | 514 (90.2) | 112 (94.1) | 97 (94.2) | 96 (93.2) | |||
| Yes | 56 (9.8) | 7 (5.9) | 6 (5.8) | 7 (6.8) | |||
| Central location | <0.001 | >0.99 | |||||
| No | 403 (70.7) | 109 (91.6) | 96 (93.2) | 96 (93.2) | |||
| Yes | 167 (29.3) | 10 (8.4) | 7 (6.8) | 7 (6.8) | |||
| Tumor location | 0.452 | 0.331 | |||||
| RUL | 148 (26.0) | 29 (24.4) | 29 (28.2) | 26 (25.2) | |||
| RML | 26 (4.6) | 5 (4.2) | 1 (1.0) | 5 (4.9) | |||
| RLL | 122 (21.4) | 33 (27.7) | 23 (22.3) | 30 (29.1) | |||
| LUL | 162 (28.4) | 26 (21.8) | 27 (26.2) | 21 (20.4) | |||
| LLL | 112 (19.6) | 26 (21.8) | 23 (22.3) | 21 (20.4) | |||
| Surgical procedure | <0.001 | >0.99 | |||||
| Lob | 400 (70.2) | 109 (91.6) | 94 (91.3) | 94 (91.3) | |||
| Others | 170 (29.8) | 10 (8.4) | 9 (8.7) | 9 (8.7) | |||
| Tumor size (mm) | 6.7±1.5 | 6.4±1.3 | 0.059 | 6.4±1.2 | 6.4±1.3 | 0.825 | |
| Pathology | 0.001 | 0.885 | |||||
| SCC | 300 (52.6) | 43 (36.1) | 37 (35.9) | 38 (36.9) | |||
| Non-SCC | 270 (47.4) | 76 (63.9) | 66 (64.1) | 65 (63.1) | |||
| Pathological T stage | 0.104 | 0.503 | |||||
| pT3 | 388 (68.1) | 90 (75.6) | 82 (79.6) | 78 (75.7) | |||
| pT4 | 182 (31.9) | 29 (24.4) | 21 (20.4) | 25 (24.3) | |||
| Pathological N stage | 0.004 | 0.347 | |||||
| pN0 | 260 (45.6) | 74 (62.2) | 62 (60.2) | 61 (59.2) | |||
| pN1 | 153 (26.8) | 21 (17.6) | 26 (25.2) | 20 (19.4) | |||
| pN2 | 157 (27.6) | 24 (20.2) | 15 (14.6) | 22 (21.4) | |||
| Surgical margin | 0.111 | 0.517 | |||||
| R0 | 528 (92.6) | 115 (96.6) | 97 (94.2) | 99 (96.1) | |||
| R1 | 42 (7.4) | 4 (3.4) | 6 (5.8) | 4 (3.9) | |||
| Grade | 0.896 | 0.664 | |||||
| Well/moderate | 346 (60.7) | 73 (61.3) | 67 (65.0) | 64 (62.1) | |||
| Poor | 224 (39.3) | 46 (38.7) | 36 (35.0) | 39 (37.9) | |||
| PI | 0.086 | 0.662 | |||||
| No | 240 (42.1) | 40 (33.6) | 38 (36.9) | 35 (34.0) | |||
| Yes | 330 (57.9) | 79 (66.4) | 65 (63.1) | 68 (66.0) | |||
| LVI | 0.462 | 0.622 | |||||
| No | 537 (94.2) | 110 (92.4) | 93 (90.3) | 95 (92.2) | |||
| Yes | 33 (5.8) | 9 (7.6) | 10 (9.7) | 8 (7.8) | |||
| ACT | 0.108 | 0.674 | |||||
| No | 300 (52.6) | 53 (44.5) | 48 (46.6) | 45 (43.7) | |||
| Yes | 270 (47.4) | 66 (55.5) | 55 (53.4) | 58 (56.3) | |||
| ART | 0.072 | >0.99 | |||||
| No | 538 (94.4) | 117 (98.3) | 101 (98.1) | 101 (98.1) | |||
| Yes | 32 (5.6) | 2 (1.7) | 2 (1.9) | 2 (1.9) | |||
Data are presented as n (%) or mean ± SD. PSM, propensity score matching; VATS, video-assisted thoracic surgery; BMI, body mass index; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lung for carbon monoxide; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; Lob, lobectomy; SCC, squamous cell carcinoma; PI, pleural invasion; LVI, lymphovascular invasion; ACT, adjuvant chemotherapy; ART, adjuvant radiotherapy; SD, standard deviation; n, number.
Intraoperative and postoperative outcomes between VATS and thoracotomy
After PSM, significantly less intraoperative blood loss was observed in the VATS group than in the thoracotomy group (111.2±83.1 vs. 206.8±390.8 mL, P=0.016). VATS approach demonstrated statistically longer operative time than thoracotomy (140.5±52.7 vs. 123.0±41.4 minutes, P=0.008). Median operation time of VATS shortened over time, from 150.8 minutes in 2013 to 130.5 minutes in 2017, while median operative time of thoracotomy remained similar, being 129.5 minutes in 2013 and 125.7 minutes in 2017 (Figure S1). VATS approach did not compromise the yield of lymph node dissection. Neither the total number of harvested lymph nodes nor the number of positive lymph nodes showed no statistical difference between the two groups. In addition, VATS resection did not increase the rate of intraoperative complications [3 (2.9%) vs. 1 (1.0%), P=0.313] in comparison with open thoracotomy (Table 2).
Table 2
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Thoracotomy (n=570) | VATS (n=119) | P | Thoracotomy (n=103) | VATS (n=103) | P | ||
| Intraoperative outcomes | |||||||
| Operative time (minutes) | 126.1±57.8 | 143.0±53.9 | 0.003 | 123.0±41.4 | 140.5±52.7 | 0.008 | |
| Harvested LNs | 14.6±6.7 | 13.3±6.4 | 0.049 | 14.5±7.4 | 13.2±6.5 | 0.177 | |
| Harvested positive LNs | 1.6±2.4 | 1.1±2.4 | 0.042 | 0.9±1.9 | 1.2±2.4 | 0.410 | |
| Blood loss (mL) | 209.2±277.0 | 127.7±115.7 | 0.002 | 206.8±390.8 | 111.2±83.1 | 0.016 | |
| Intraoperative complication | 19 (3.3) | 9 (7.6) | 0.034 | 3 (2.9) | 1 (1.0) | 0.313 | |
| Postoperative outcomes | |||||||
| LOS (days) | 9.4±12.5 | 7.3±5.4 | 0.085 | 8.0±3.8 | 7.2±5.4 | 0.197 | |
| Chest drainage duration (days) | 6.5±12.4 | 5.3±5.1 | 0.290 | 5.2±3.9 | 5.1±5.1 | 0.830 | |
| Postoperative complications | 73 (12.8) | 16 (13.4) | 0.180 | 10 (9.7) | 12 (11.7) | 0.652 | |
| In-hospital mortality | 4 (0.7) | 1 (0.8) | 0.877 | 0 | 1 (1.0) | 0.316 | |
Data are presented as n (%) or mean ± SD. PSM, propensity score matching; VATS, video-assisted thoracic surgery; LN, lymph node; LOS, length of hospital stay; SD, standard deviation; n, number.
As for postoperative outcomes, VATS did not compromise the postoperative safety and feasibility in comparison with open thoracotomy before or after PSM. There was no significant difference between the VATS and thoracotomy groups in LOS, chest tube duration, rate of postoperative complications, or in-hospital mortality (Table 2).
For detailed postoperative complications, VATS presented similar outcomes including prolonged air leakage, arrhythmia, postoperative bleeding, BPF, chylothorax, pulmonary infection or pyothorax, pleural effusion, reoperation, readmission and other complications in accordance with traditional thoracotomy either before or after PSM (Table S1).
Oncological outcomes
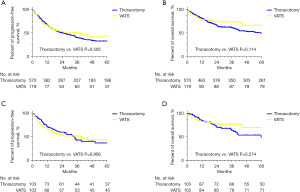
The median follow-up was 42.1 months (0 to 73.9 months). A total of 264 patients (38.3%) experienced cancer recurrence included local relapse or distant metastasis, and 169 patients (24.5%) died within the follow-up interval. Five-year PFS was similar after VATS (42.6%) and thoracotomy (33.0%, P=0.505) and the 5-year OS was similar after VATS (66.1%) and thoracotomy (49.3%, P=0.114), respectively (Figure 2A,2B). After PSM, Five-year PFS was similar after VATS (43.4%) and thoracotomy (36.5%, P=0.956) and the 5-year OS was similar after VATS (68.7%) and thoracotomy (49.1%, P=0.274), respectively (Figure 2C,2D).
In univariable analysis, VATS approach was not associated with either PFS or OS. Pathological N stage, cancer differentiation grade and PI were found to be associated with recurrence. Further, patient age and cancer differentiation grade were also associated with OS (Table 3). In multivariable analysis, pathological N2 stage [hazard ratio (HR) =2.093, 95% confidence interval (CI): 1.263–3.469, P=0.004] and PI (HR =1.808, 95% CI: 1.132–2.886, P=0.013) were independently correlated with shorter PFS. Older age (HR =1.065, 95% CI: 1.026–1.106, P=0.001) and poor differentiation (HR =2.313, 95% CI: 1.353–3.952, P=0.002) were independently correlated with worse OS (Table 3).
Table 3
| Variables | Progression-free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||
| P | HR (95% CI) | P | P | HR (95% CI) | P | ||||
| Gender (female vs. male) | 0.605 | – | – | 0.957 | – | – | |||
| Age (per years) | 0.081 | – | – | 0.001 | 1.065 (1.026–1.106) | 0.001 | |||
| Smoking history (yes vs. no) | 0.503 | – | – | 0.654 | – | – | |||
| BMI (per kg/m2) | 0.595 | – | – | 0.857 | – | – | |||
| FEV1% (per %) | 0.126 | – | – | 0.066 | – | – | |||
| DLCO% (per %) | 0.306 | – | – | 0.256 | – | – | |||
| Comorbidity (yes vs. no) | 0.782 | – | – | 0.710 | – | – | |||
| Central location (yes vs. no) | 0.388 | – | – | 0.985 | – | – | |||
| Tumor location (vs. RUL) | |||||||||
| RML | 0.130 | – | – | 0.150 | – | – | |||
| RLL | – | – | – | – | |||||
| LUL | – | – | – | – | |||||
| LLL | – | – | – | – | |||||
| Surgical procedure (others vs. Lob) | 0.470 | – | – | 0.741 | – | – | |||
| Tumor size (per mm) | 0.906 | – | – | 0.193 | – | – | |||
| Pathology (non-SCC vs. SCC) | 0.240 | – | – | 0.748 | – | – | |||
| Pathological T stage (pT4 vs. pT3) | 0.464 | – | – | 0.830 | – | – | |||
| Pathological N stage (vs. pN0) | |||||||||
| pN1 | 0.003 | 1.197 (0.706–2.030) | 0.504 | 0.443 | – | – | |||
| pN2 | 2.093 (1.263–3.469) | 0.004 | – | – | |||||
| Surgical margin (R1 vs. R0) | 0.722 | – | – | 0.891 | – | – | |||
| Grade (poor vs. well/moderate) | 0.026 | 1.311 (0.853–2.016) | 0.217 | 0.002 | 2.313 (1.353–3.952) | 0.002 | |||
| PI (yes vs. no) | 0.008 | 1.808 (1.132–2.886) | 0.013 | 0.055 | – | – | |||
| LVI (yes vs. no) | 0.542 | – | – | 0.569 | – | – | |||
| ACT (yes vs. no) | 0.731 | – | – | 0.100 | – | – | |||
| ART (yes vs. no) | 0.556 | – | – | 0.477 | – | – | |||
| Surgical approach (VATS vs. open) | 0.956 | – | – | 0.276 | – | – | |||
PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lung for carbon monoxide; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; Lob, lobectomy; SCC, squamous cell carcinoma; PI, pleural invasion; LVI, lymphovascular invasion; ACT, adjuvant chemotherapy; ART, adjuvant radiotherapy; VATS, video-assisted thoracic surgery.
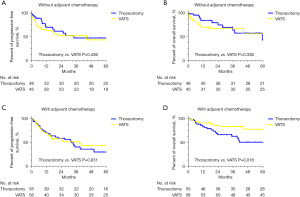
Further, we performed the subgroup analysis according to the status of postoperative chemotherapy. In the subgroup of patients without receiving ACT, we found that the VATS group demonstrated comparable 5-year PFS and OS in comparison with the thoracotomy group (Figure 3A,3B). VATS approach showed better 5-year OS rate than thoracotomy (78.1% vs. 50.8%, P=0.018) and similar PFS rate among those patients receiving ACT when against open thoracotomy (Figure 3C,3D).
Subgroup analysis of conversion to thoracotomy
There were eight cases converted to thoracotomy during VATS resections, four because of intraoperative massive hemorrhage, two of extensive tumor invasion and two of dense adhesion. The conversion cases had older age (71.8±8.4 vs. 63.8±9.1 years, P=0.017) and more central lesions (37.5% vs. 6.3%, P=0.002) than VATS (Table S2). Operative time (187.8±37.8 vs. 139.7±53.5 minutes, P=0.014) was significantly longer and intraoperative blood loss significantly more (362.5±231.1 vs. 110.9±81.6 mL, P<0.001) than those of the VATS group. Conversion to thoracotomy had statistically longer chest drainage duration (8.8±5.6 vs. 5.0±5.0 days, P=0.047) and longer LOS (10.5±6.0 vs. 7.1±5.3 days, P=0.085) than VATS approach. But ability of lymph node dissection, rate of postoperative complications, or in-hospital mortality were similar between the conversion and VATS groups (Tables S3,S4). In univariable and multivariable logistic analyses, older age [odds ratio (OR) =5.934, 95% CI: 1.029–34.207, P=0.046] and centrally located lesion (OR =1.117, 95% CI: 1.001–1.248, P=0.049) were independently correlated with higher risk of conversion to open thoracotomy (Table S5).
Discussion
The size of NSCLC larger than 5 cm, which is classified into T3 and T4 stage tumors, is considered as locally advanced diseases. Surgical resection plays a role in the treatment of those T3 and resectable T4 NSCLCs, though radical resection is complicated (2,12). VATS resection has been tried to broaden from the early-stage NSCLC to primary NSCLC which was large than 5 cm in diameter according to previous study (13). The short-term clinical outcomes of VATS have been well-clarified among early-stage NSCLCs, but the safety and feasibility of VATS in NSCLC larger than 5 cm remained unclear (10,14-18). In 2016, Dr. Chen and his teammates pointed out that VATS lobectomy could be of safety with acceptable clinical outcomes for those LA-NSCLC (19). However, this study enrolled clinical T1 NSCLC into statistical analysis and it might not be convincing for the application of VATS resection to those NSCLC larger than 5 cm according to the 7th TNM classification of lung cancer. In addition, tumor size larger than 5 cm is reclassified into T3 and T4 stage in accordance with the 8th TNM classification and the related researches are still limited.
In this study, we enrolled 689 patients, the largest cohort to now specifically focused on NSCLC larger than 5 cm receiving upfront VATS and open thoracotomy. After 1:1 PSM, we found that VATS was an alternative surgical approach worthy attempted. VATS was safe and feasible in NSCLC larger than 5 cm, with less blood loss, low conversion rate, similar intraoperative and postoperative complication rate, and similar complete resection rate compared to thoracotomy. Though operative time was longer in the VATS group, the gap got shorter as time went on. And VATS improved OS of patients with NSCLC larger than 5 cm receiving ACT, but had no association with PFS or OS in those without ACT.
Surgery for NSCLC larger than 5 cm is more complicated. Large tumor size leads to limited operative space, and is more likely to be companied with invasion of bronchus, vessels and lymph nodes as LA-NSCLC. In our study, only 17.3% (119/689) cases were performed by VATS. The operative time was long, with 123.0±41.4 minutes in the thoracotomy group and 140.5±52.7 minutes in the VATS group (P=0.008). However, rate of VATS got higher and time of operation got shorter over time, especially for VATS (Figure S1). In 2013, the median operative time was 129.5 and 150.8 minutes in the thoracotomy and VATS groups respectively. In 2017, it was 125.7 and 130.5 minutes in the thoracotomy and VATS groups.
Apart from operative time, perioperative clinical results of the VATS group were no worse than thoracotomy. For intraoperative outcomes, VATS presented much less volume of blood loss, similar intraoperative complications and comparable ability of lymph node dissection over the thoracotomy, owing to the precise anatomy and operation that VATS given to us thoracic surgeons. For postoperative feasibility and safety, length of stay was shorter in the VATS group than the thoracotomy group (7.3±5.4 vs. 9.4±12.5 days, P=0.085) with borderline P value. Chest tube duration, postoperative complication rate and the rate of in-hospital mortality were similar between the VATS and thoracotomy groups. In detail, the VATS group had similar rate of prolonged air leakage and bleeding, along with comparable arrhythmia and pulmonary infection rate against thoracotomy. More importantly, there was no case of BPF, chylothorax, large volume of pleural effusion, reoperation, readmission or other complications in the VATS group. Those perioperative results mentioned above were just in same with Dr. Nakano’s research and previous results (13,20).
Second is the low conversion rate and acceptable clinical outcomes of the conversion subgroup. The conversion rate (7.8%, 8/103) is totally comparable against that of early-stage NSCLC, which was 5.7% (14/246) (21). Conversion cases were much older patients with relatively aggressive tumor status compared with those of the VATS group. Therefore, longer operative time as well as worse survival outcome was foreseeable under this selection bias. Apart from operative time and blood loss, conversion cases still demonstrated no difference on other perioperative data with the VATS group, which was in accordance with previous research (22). All these results also reflected the significant safety and feasibility of VATS approach from another aspect.
Third, the rate of R0 resection in the VATS group was similar to those in the thoracotomy group, which strongly indicated that the surgical approaches did not influence the surgically complete resection outcomes. Hence, with the preferable short-term outcomes, low conversion rate and comparable rate of R0 resection against the open group, though with difficulty, VATS could be accepted as a safe and feasible surgery a1pproach for LA-NSCLC.
It has been well-documented that VATS resection benefits patients with better long-term survival outcomes for early-stage NSCLCs than thoracotomy, probably owing to more precise surgical resection, less postoperative pain, earlier recovery (23,24). However, the survival benefit of VATS for NSCLCs larger than 5 cm is still limited. Dr. Bu indicated that complete VATS was similar to open approach in safety, completeness and efficacy, and had a shorter operative time and reduced bleeding among NSCLC with tumor size >5 cm. Nevertheless, long-term survival outcomes were not thoroughly mentioned (9). Dr. Nakano had pointed out the similar survival outcomes for both VATS and thoracotomy among patients with lung cancer >5 cm. However, only 68 patients were enrolled in statistical analysis (13).
In our analysis, we totally enrolled 689 qualified patients. It was found that VATS resection presented obviously improved OS and similar PFS in subgroup of patients receiving ACT compared with thoracotomy after adjustment. In subgroup of patients without ACT, VATS approach presented similar PFS and OS compared with thoracotomy either before or after variable matching. VATS had no impact on oncological results as this surgical approach did not influence R0 rate. But VATS brought less trauma to the whole body. Thoracotomy together with followed ACT might influence OS as thoracotomy and chemotherapy both brought trauma to patients to a certain extent. When we replaced thoracotomy by a less invasive approach, like VATS, less trauma might lead to OS benefits. VATS could be an alternative surgical approach for those LA-NSCLCs larger than 5 cm, with potentially survival benefit for patients receiving ACT.
However, there were still several limitations in this research. First, it was a retrospective analysis, the data were all collected from one single institution. Although variables were adjusted through the PSM, selection biases were still naturally unavoidable. Multicentric randomized clinical trial are required to validate the conclusion. Second, the follow-up time of our study was not adequate. It still needed much more time to follow up oncological prognosis and enhance the power of evidence. Third, patients with induction therapy were excluded to our study. Actually, large amounts of LA-NSCLC patients have been receiving neoadjuvant therapy, such as preoperative chemotherapy, targeted therapy or immunotherapy in recent years. Therefore, investigating the safety and feasibility for VATS resection among LA-NSCLC patients after induction therapy should become our priority in the next step. Moreover, it is truly a challenge for thoracic surgeon to complete a LA-NSCLC surgery under the VATS approach and the learning curves are relatively longer.
Conclusions
In conclusions, upfront surgery of VATS approach presented reliable safety and feasibility for patients with NSCLC larger than 5 cm compared with thoracotomy. Perioperative benefits were likely to be gained over time. VATS not only showed comparable PFS and OS in total in accordance with thoracotomy, but significantly benefited patients receiving ACT with better OS outcomes. Therefore, VATS could be considered as a safe and feasible choice for those well-selected patients with NSCLC larger than 5 cm.
Acknowledgments
The research group thanks all the patients participating in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-39/rc
Data Sharing Statement: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-39/dss
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-39/coif). W.F. serves as the unpaid Executive Editor-in-Chief of Shanghai Chest. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Chest Hospital (No. IS21113) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Robinson BM, Kennedy C, McLean J, et al. Node-negative non-small cell lung cancer: pathological staging and survival in 1765 consecutive cases. J Thorac Oncol 2011;6:1691-6. [Crossref] [PubMed]
- Shien K, Toyooka S, Kiura K, et al. Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer. Ann Surg Oncol 2012;19:2685-92. [Crossref] [PubMed]
- DiPerna CA, Wood DE. Surgical management of T3 and T4 lung cancer. Clin Cancer Res 2005;11:5038s-44s. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Dziedzic D, Orlowski T. The Role of VATS in Lung Cancer Surgery: Current Status and Prospects for Development. Minim Invasive Surg 2015;2015:938430. [Crossref] [PubMed]
- Bu L, Li Y, Yang F, et al. Completely video-assisted thoracoscopic lobectomy versus open lobectomy for non-small cell lung cancer greater than 5 cm: a retrospective study. Chin Med J (Engl) 2012;125:434-9. [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Ikeda N. Updates on Minimally Invasive Surgery in Non-Small Cell Lung Cancer. Curr Treat Options Oncol 2019;20:16. [Crossref] [PubMed]
- Daly BD, Ebright MI, Walkey AJ, et al. Impact of neoadjuvant chemoradiotherapy followed by surgical resection on node-negative T3 and T4 non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;141:1392-7. [Crossref] [PubMed]
- Nakano T, Endo S, Endo T, et al. Surgical Outcome of Video-Assisted Thoracoscopic Surgery vs. Thoracotomy for Primary Lung Cancer >5 cm in Diameter. Ann Thorac Cardiovasc Surg 2015;21:428-34. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-6. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-976.e2. [Crossref] [PubMed]
- Bailey KL, Merchant N, Seo YJ, et al. Short-Term Readmissions After Open, Thoracoscopic, and Robotic Lobectomy for Lung Cancer Based on the Nationwide Readmissions Database. World J Surg 2019;43:1377-84. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid 2022; [Crossref]
- Fourdrain A, De Dominicis F, Iquille J, et al. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure†. Eur J Cardiothorac Surg 2019;55:660-5. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Dziedzic R, Marjanski T, Binczyk F, et al. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2018;54:547-53. [Crossref] [PubMed]
Cite this article as: Wang Y, Chen Y, Wang J, Chen T, Bao F, Gu Z, Fang W. Video-assisted thoracoscopic surgery for patients with non-small cell lung cancer larger than 5 cm. Shanghai Chest 2024;8:2.


