
Postoperative pulmonary compensation after lung cancer surgery: a shift towards a modern and comprehensive model—a narrative review
Introduction
Lung cancer is the second most common neoplasm and the first cause of cancer-related death in both sexes with a projected increasing incidence, especially in women (1,2). A straightforward surgery is mandatory for early-stage non-small cell lung cancer (NSCLC) and pulmonary lobectomy represents the gold standard approach for radical therapy (3). However, anatomical resections result in permanent tissue loss and postoperative impairment of pulmonary function. Although ancestral thoughts held this loss was directly proportional to the volume of resected parenchyma, evidence has suggested a new concept of postoperative remodelling adaptation, overcoming the exclusive anatomical theory of vicarious compensation. Moreover, variability in medium- and long-term functionality can be not justified by a mere hypertrophic-volumetric model without taking in account postoperative air dynamic variations within the tracheobronchial tree, such as changes in flow rate and redistribution, pressure, and wall shear stress.
The aim of this unsystematic narrative review is to highlight correlations between morpho-dynamic changes of the tracheobronchial tree and the onset of complex steno-obstructive clinical scenarios in patients undergoing minimally invasive or open pulmonary lobectomy. We present this article in accordance with the Narrative Review reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-26/rc).
Methods
An unsystematic narrative review of published articles about post-operative pulmonary compensation after lobectomies for NSCLC was conducted.
Sources of information
Research was done by accessing the following databases: PubMed-MEDLINE and Scopus.
Delimiting search terms
Delimiting search MeSH terms were: pulmonary compensation or remodel; lung cancer; surgery; post-operative pulmonary function; anatomic changes; lung mechanics; ventilation; one-lung ventilation; post-operative pulmonary tests.
Selection criteria
Inclusion criteria were: papers written in English about post-resectional anatomic and dynamic pulmonary compensation [2010–2023] (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Last update: May 31,2023 |
| Databases and other sources searched | PubMed, Scopus |
| Search terms used | Pulmonary compensation or remodel; lung cancer; surgery; post-operative pulmonary function; anatomic changes; lung mechanics; ventilation; one-lung ventilation; post-operative pulmonary tests |
| Timeframe | 2010–2023 |
| Inclusion criteria | Papers written in English about post-resectional anatomic and dynamic pulmonary compensation |
| Selection process | Three researches, independently |
Discussion
Postoperative anatomical modifications of the tracheobronchial tree
Lobectomies induce postoperative adaptive changes in the pleural cavity and chest wall (4). After left upper lobectomy, left main bronchus distants in a sigmoidal pattern, as consequence of an anatomical remodelling resulting from an upward displacement of the ipsilateral hemidiaphragm and of the remaining inferior lobe with concomitant twisting of secondary bronchi and benting downstream the sutured bronchial stump (5).
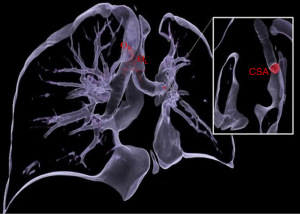
Moreover, variations in the angular incidence and ventilatory surface of the tracheobronchial tree contribute to morphological alterations. In fact, as a synergistic effect to anatomical adaptive processes, the in-plane bifurcation angles between the trachea and the main bronchi, as well as the cross-sectional area (CSA) of the remaining ipsilateral lobar bronchus, undergo substantial structural changes. In the case of upper lobectomies, the incident angle of the tracheobronchial axis decreases on the resected lung. Gu et al. (6), in a single-centre retrospective analysis including 18 patients undergoing left upper video-assisted thoracoscopic surgery (VATS) lobectomy, reported a significant reduction of the left main bronchial angle (ΔƟ left =−13.4°, P<0.01) at the expense of an equal increase of the contralateral one (ΔƟ right =+10.5°, P<0.01).
Increases in right ΔƟ were also observed in right lower lobectomies with an average gain of 10.94%, while in left lower procedures, geometrical alterations of the remaining lobe appear less marked. With respect to the CSA, measured at the middle point of the remaining lobar lobe, it is significantly inferior in all lobectomies, indicating a tendency to narrow and predisposing to compensatory stenosis with a consequent alteration of fluid dynamic parameters (7).
In general, the greatest reduction is observed in upper lobectomies and, in particular, in left ones with a median CSA decrease ranging from 10–75% (6,7). Non-significant reductions are also reported in lower lobectomies demonstrating postoperative rotation vectors substantially do not affect the incident angle of the origin of the residual upper lobar bronchus (particularly for right lower cases where effects are presumably mitigated by the pin action of the bronchus intermedius stump) (Figure 1).
Airflow and fluid dynamics alterations
Regardless of the extent and laterality of the resected lobe, changes in the fluid dynamic parameters can be found in the post-operative period.
Even if inlet flow area is equal, there is a substantial increase in the flow velocity, wall pressure, wall shear stress, and pressure drop causing rearrangement of the airflow rate.
In general, post-operative geometries are subject to a statistically significant increase in flow velocity in the entire tracheobronchial tree with significant increases in the case of left upper lobectomies due to the severe reduction of the CSA of the bronchus downstream the resection and determining a high-velocity gradient comparable to the carinal peak value with transmission up to the smallest sublobar bronchial generations (8).
In terms of pressure, maximum peak values are observable postoperatively in the trachea and main bronchi and the entire tracheobronchial tree appears to be subject to an augmented pressure increase in relation to a constant relationship between the pressure drop and the inlet area suggesting an increase of airway resistance (8).
Therefore, there is a generalized worsening of aerodynamic conditions and the increase in flow proportionally remodulates both wall shear stress up to the second bronchial (lobar) generations and lobar airflow rates. Conversely, the right lung receives the greatest redistribution in flow (9).
Physiopathology of compensatory adaptation
Complex postoperative structural and dynamic mechanisms in the tracheobronchial tree translate into pulmonary pathophysiological changes as the result of postoperative compensation and promoting several clinical scenarios. Historically, parenchymal adaptation was attributed to a mere proportional increase in residual lung tissue and to a proportional increase in residual ipsilateral lobar ventilation-perfusion ratio (10,11).
However, the concept of post-lobectomy redistribution cannot be justified by a simple pulmonary hyperinflative action on preexisting septal alveolar tissue; rather, it represents the epiphenomenon of a complex cooperation between recruitment of alveolar capillary reserves and static and dynamic remodelling of the airways, being the volumetric increase only a functional aspect not justifying clinical variability in lobectomized patients.
The role of growth factors has been implicated in this process. Experimental models have shown that the induction of leukocyte chemotaxis in the residual lung parenchyma causes a transient increase in mitogenic activity lasting few months after lung resection (12,13). Regarding the effect of perioperative IGF-1/IGFBP-3, serum concentrations on capillary proliferation and angiogenesis (14), a meta-analysis has demonstrated a high incretory variability due to various inhibiting factors, such as aging-related decrease in titers of about 14% per decade of life (15,16).
The extent of alveolar capillary remodeling can be deduced proportionally from changes in post-operative respiratory function and, in particular, from the analysis of fluctuations of the diffusing lung capacity for carbon monoxide (DLCO) and of its ratio with total alveolar volume.
Yokoba et al. (17), in a prospective study enrolling 59 NSCLC patients undergoing VATS lobectomy, reported a significant drop in DLCO 3 months after surgery with a trend towards a progressive recovery at 6 and 12 months. Conversely, DLCO/alveolar volume did not change for any type of pulmonary resection, except for 3 months after right upper lobectomy (preoperative, 3-, 6-, 12-months DLCO/alveolar volume: 102.62±23.88, 94.95±20.51, 98.56±22.42 and 98.87±22.54, respectively; P<0.003). The results suggest a substantial absence of significant changes in gas diffusibility at the alveolar-capillary interface and an adaptive increase in the capillary bed due to early changes in pulmonary hemodynamics under conditions of a reduced pulmonary volume after surgery.
Although stereologically Sengul et al. (18), in a retrospective study including 30 patients undergoing anatomical lung resection, showed a significant difference in lung volume loss, especially for upper lobectomies (up to 19.01% of the total volume) and a proportional decrease in postoperative forced vital capacity (FVC), the effects on pulmonary function tests (PFTs) cannot fail to take into account also favorable and unfavorable post-operative morpho-dynamic alterations.
Favorable adaptations include an increase in the contralateral tracheobronchial angle incidence and air-flow rate ratio, responsible for an early ventilatory redistribution. At the same time, unfavourable changes in respiratory dynamics can be attributed to ipsilateral angle decrease and to vicarious distortion of main bronchus secondary to displacement of the hemidiaphragm and to mechanisms of obliteration of the residual pleural cavity, as an epiphenomenon of anatomic rearrangement causing ipsilateral mediastinal shift with possible dynamic compression on right heart or the appearance of apical left atrial swirling vortices due to venous pulmonary stump stagnating blood flow (19,20). Furthermore, a significant reduction of the bronchial CSA results into an increased turbulent flow velocity, endobronchial pressure, wall shear stress and pressure drop, in particular in the case of upper lobectomies, worsening the occurrence of postoperative bronchial kinking and of postoperative pulmonary complications, such as persistent cough, dyspnea, bronchomalacia (5,8,20).
In this regard, the adaptation of the middle lobe after a right upper lobectomy with detrimental post-operative parenchymal functions due to atelectasis is explanatory (21). In a single-center case series, lobar kinkings in 12% of patients were reported as a result of a synergistic effect between anatomical peculiarities and fluid-dynamic compensations (22). On the other hand, the same adaptive mechanisms of the middle lobe appear protective in the case of right lower lobectomies. Yamaghishi et al. (23), retrospectively analyzing 53 patients undergoing surgery for living-donor lobar lung transplantation and evaluating the morphometric changes, parenchymal fractal dimensional variations and respiratory volumetric fluctuations, demonstrated compensatory morphological and functional reserves in the middle lobe both in the static (130.9%±19.7%) and in the functional lobar volume (97.2±73.5 mL).
Similarly, anatomic pulmonary segmentectomies also promote adaptive residual lobar and ipsilateral pulmonary excursions. Tane et al. (24) clearly showed that volumetric preservation was positively correlated to the extent of resected segments (1-segment vs. >1-segment resections: 84.6% vs. 66.1%; P<0.01) without significant differences between typical and segmentectomies atypical. However, enrolling 148 matched patients undergoing VATS pulmonary resections (74 segmentectomies vs. 74 lobectomies), the authors showed a greater residual lobar functional preservation after S6 segmentectomies. On the other hand, left upper division segmentectomies, ontogenically comparable to a right upper lobectomy, resulted in a reduced parenchymal function due to segmental lingural bronchial displacement and kinking with reduced postoperative forced expiratory volume in 1 second (FEV1).
Clinical aspects and management
Pulmonary adaptive compensation lays the foundation for understanding the aetiology of static and dynamic steno-obstructive symptoms in the short- and long-term period in patients undergoing minimally invasive or open pulmonary lobectomy.
The dynamics of the residual displaced lung and vicariate rotation actions could cause bronchial kinking and worsening postoperative pulmonary functions. Although generally subclinical, as reported by Ueda et al. (10), the incidence reaches 42% of patients undergoing upper lobectomies resulting in a significant reduction of postoperative flow volume loop (FVL) and ventilatory capacity (VC) (P<0.05) and in a reduced efficacy of favorable compensatory mechanisms by promoting the onset of chronic obstructive symptoms, as well as a ventilatory mismatch due to an increase in airway resistance and functional residual volume (FRV) which, in turn, adversely affect patients’ quality of life. Clinically, it manifests as postoperative shortness of breath, dyspnea, impaired muco-ciliary clearance, chronic cough, and pneumonia (14) and it significantly influences postoperative rehabilitation (25).
The role of prophylactic intraoperative maneuvers (e.g., inferior pulmonary ligament division) aiming to mitigate tendencies for postoperative stenosis remains uncertain and debated. Kuriyama et al. (26), in a recent retrospective study enrolling 213 upper lobectomy patients, clearly demonstrated a statistically unsignificant difference in postoperative complications, FVC, and FEV1 by preserving the inferior pulmonary ligament during right upper lobectomies; however, PFTs were significantly better after left lobectomies, but, no substantial variations in terms of CSA and residual ipsilateral bronchial circumference between sides was reported.
Furthermore, long-term effects of an increased lobar or pulmonary flow rate, bronchial pressure, as well as wall shear stresses could result in airway trauma, reverberating an inflammatory response sustained by an overexpression of inflammatory mediators and leukocyte activation leading to deterioration and remodelling of the alveolar glycocalyx (27).
In the context of surgical outcomes for lung cancer, a consistent risk for a second synchronous or metachronous tumour has been reported, with incidences up to 28.4% (28), and some has claimed for the need of a reoperation on an anatomically and functionally altered tracheobronchial tree (29).
Oxygen mismatches, the lack of functional lung parenchyma and altered static and dynamic adaptive mechanisms could rapidly precipitate intraoperatively in critical hypercapnia. In fact, the reduced adult lung parenchymal plasticity and residual exercise intolerance due to post-surgical hemodynamic changes could collide with anaesthetic challenges, despite permissive PFTs.
When dealing with previous lung resection and the need for contralateral reoperation, several factors can destabilise the precariousness of a cardiopulmonary balance during one-lung ventilation (OLV). In fact, tidal volumes would be released to a remodelled lung parenchyma unable for maximal alveolar expansion and, thus theoretically, predisposing to overinflation and barotrauma leading to breath-stacking, a build-up of intrinsic positive end-expiratory pressure (PEEP) and increased alveolar vascular resistances increasing dead space and inefficient gas exchanges with a ventilation-perfusion ratio greater than 1. Increased inspiratory plateau pressures would play a synergistic role with high intrapulmonary vascular resistances as well as augmented right ventricular afterload contributing to a reduction in left ventricular compliance and cardiac output, precipitating hemodynamic compromise in response to a previous pulmonary resection (30,31).
In this scenario, the anatomical concept of compensatory pulmonary adaptation does not fully fit with risks of intraoperative hypoxemia in the setting of a reoperation, laying the foundation to a dynamic ventilation-perfusion model, where the effects of the hypoxemic pulmonary vasoconstriction and the inversion of the ventilation/perfusion ratio are attenuated by recruitability of pulmonary vessels in the dependent lung mitigating the fall in diffusion capacity and shunt-ratio. Moreover, the increase of the dependent intrapleural pressure could lead to a further reduction of functional residual capacity, claiming for higher inspiratory pressures and PEEP levels to deliver tidal volume and maintain driving pressure unchanged (31,32).
Moreover, intraoperative recruitment maneuvers, such as continuous positive airway pressure (CPAP), intermittent positive airway pressures or lobar isolation techniques with bronchial blockers to support OLV in patients with prior contralateral pulmonary resections would be needed (31,33).
Ventilation is similarly affected by a previous lobectomy on the dependent lung. Protective lung ventilation strategies with PEEP titration are crucial given the minimal residual volumetric amount of the lung parenchyma. Essential component for a strategic intraoperative management is a correct tidal volume delivery, since CO2 elimination is directly influenced by both the proportion of shunt fractions and increased unventilated alveolar surfaces (34).
Finally, the modulation of the respiratory rate would not guarantee compensation and, in the case of reoperation on the contralateral lung, it would favor an increase in dynamic hyperinflation and in inspiratory airway pressures which could expose to the risk of alveolar stress injury.
Conclusions
Postoperative pulmonary adaptive response involves morpho-structural changes influencing postoperative respiratory function and medium-long term sequelae in surgically treated patients with NSCLC. The mechanisms of remodulation of the residual lung volume correspond to dynamic compensations of air-flow redistribution at the expense of an increase in pressure and shear stress into the tracheobronchial tree. Finally, compensation represents a remodulation resource in response to surgical stress as the epiphenomenon of a complex cooperation between the host response, recruitment of alveolar-capillary reserves and fluid dynamics remodulation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-26/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-26/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Luo G, Zhang Y, Etxeberria J, et al. Projections of Lung Cancer Incidence by 2035 in 40 Countries Worldwide: Population-Based Study. JMIR Public Health Surveill 2023;9:e43651. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Kang YR, Kim JS, Cha YK, et al. Imaging findings of complications after thoracic surgery. Jpn J Radiol 2019;37:209-19. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Clinical ramifications of bronchial kink after upper lobectomy. Ann Thorac Surg 2012;93:259-65. [Crossref] [PubMed]
- Gu Q, Qi S, Yue Y, et al. Structural and functional alterations of the tracheobronchial tree after left upper pulmonary lobectomy for lung cancer. Biomed Eng Online 2019;18:105. [Crossref] [PubMed]
- Aliboni L, Tullio M, Pennati F, et al. Functional analysis of the airways after pulmonary lobectomy through computational fluid dynamics. Sci Rep 2022;12:3321. [Crossref] [PubMed]
- Seok Y, Cho S, Lee JY, et al. The effect of postoperative change in bronchial angle on postoperative pulmonary function after upper lobectomy in lung cancer patients. Interact Cardiovasc Thorac Surg 2014;18:183-8. [Crossref] [PubMed]
- Evans DJ, Green AS, Thomas NK. Wall shear stress distributions in a model of normal and constricted small airways. Proc Inst Mech Eng H 2014;228:362-70. [Crossref] [PubMed]
- Ueda K, Hayashi M, Tanaka N, et al. Long-term pulmonary function after major lung resection. Gen Thorac Cardiovasc Surg 2014;62:24-30. [Crossref] [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [Crossref] [PubMed]
- Choi H, Hwang W. Perioperative Inflammatory Response and Cancer Recurrence in Lung Cancer Surgery: A Narrative Review. Front Surg 2022;9:888630. [Crossref] [PubMed]
- Duan X, Zhu Y, Cui Y, et al. Circulating tumor cells in the pulmonary vein increase significantly after lobectomy: A prospective observational study. Thorac Cancer 2019;10:163-9. [Crossref] [PubMed]
- McAnulty RJ, Guerreiro D, Cambrey AD, et al. Growth factor activity in the lung during compensatory growth after pneumonectomy: evidence of a role for IGF-1. Eur Respir J 1992;5:739-47. [Crossref] [PubMed]
- Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53. [Crossref] [PubMed]
- Wolk A. The growth hormone and insulin-like growth factor I axis, and cancer. Lancet 2004;363:1336-7. [Crossref] [PubMed]
- Yokoba M, Ichikawa T, Harada S, et al. Postoperative pulmonary function changes according to the resected lobe: a 1-year follow-up study of lobectomized patients. J Thorac Dis 2018;10:6891-902. [Crossref] [PubMed]
- Sengul AT, Sahin B, Celenk C, et al. Postoperative lung volume change depending on the resected lobe. Thorac Cardiovasc Surg 2013;61:131-7. [Crossref] [PubMed]
- Nonaka M, Kadokura M, Yamamoto S, et al. Analysis of the anatomic changes in the thoracic cage after a lung resection using magnetic resonance imaging. Surg Today 2000;30:879-85. [Crossref] [PubMed]
- Nakano T, Kaneda H, Murakawa T. Stagnating blood flow related to thrombus formation in pulmonary vein stump after left upper lobectomy. Gen Thorac Cardiovasc Surg 2023;71:648-56. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. A segmentectomy of the right upper lobe has an advantage over a right upper lobectomy regarding the preservation of the functional volume of the right middle lobe: analysis by perfusion single-photon emission computed tomography/computed tomography. Surg Today 2010;40:614-9. [Crossref] [PubMed]
- Masuda Y, Marutsuka T, Suzuki M. A risk factor for kinked middle lobar bronchus following right upper lobectomy. Asian Cardiovasc Thorac Ann 2014;22:955-9. [Crossref] [PubMed]
- Yamagishi H, Chen-Yoshikawa TF, Oguma T, et al. Morphological and functional reserves of the right middle lobe: Radiological analysis of changes after right lower lobectomy in healthy individuals. J Thorac Cardiovasc Surg 2021;162:1417-1423.e2. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the Residual Lung Function After Thoracoscopic Segmentectomy Compared With Lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149-55. [Crossref] [PubMed]
- Kuriyama S, Imai K, Saito H, et al. Inferior pulmonary ligament division during left upper lobectomy causes pulmonary dysfunction. Interdiscip Cardiovasc Thorac Surg 2023;36:ivad035. [Crossref] [PubMed]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. [Crossref] [PubMed]
- Mucilli F, Camplese P, Cipollone G, et al. Second Primary Lung Cancer: A Current Problem in Long-Survivor Cancer Patients. J J Cancer Sci Res 2016;2:028.
- Jiang L, He J, Shi X, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer 2015;87:303-10. [Crossref] [PubMed]
- Marseu K, Slinger P, de Perrot M, et al. Dynamic hyperinflation and cardiac arrest during one-lung ventilation: a case report. Can J Anaesth 2011;58:396-400. [Crossref] [PubMed]
- Ruiz P, Kovarik G. Lung mechanics and gas exchange in one-lung ventilation following contralateral resection. Can J Anaesth 2005;52:986-9. [Crossref] [PubMed]
- Licker M, Hagerman A, Jeleff A, et al. The hypoxic pulmonary vasoconstriction: From physiology to clinical application in thoracic surgery. Saudi J Anaesth 2021;15:250-63. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Durkin C, Lohser J. Oxygenation and Ventilation Strategies for Patients Undergoing Lung Resection Surgery After Prior Lobectomy or Pneumonectomy. Curr Anesthesiol Rep 2016;6:135-41. [Crossref]
Cite this article as: Barone M, Frontera R, Liouras RV, Guetti L, Dell’Atti I, Vetrugno L, Mucilli F, Maggiore SM. Postoperative pulmonary compensation after lung cancer surgery: a shift towards a modern and comprehensive model—a narrative review. Shanghai Chest 2024;8:5.


