
Morphology and immunohistochemical and molecular markers for diagnosis and guiding therapy in mesothelioma: a narrative review
Introduction
Outside of large referral centers, mesothelioma is rarely encountered by the general surgical pathologist, with only around 2,400–3,200 cases per year in the United States, a number which has largely stabilized, but increasing numbers of cases are being reported in other parts of the world, including China (1-3). Mesothelioma is often diagnosed by medical thoracoscopy which has a high diagnostic yield (4-7). In contrast to carcinomas of the lung, which have long been well characterized both immunophenotypically and genetically, use of immunohistochemistry (IHC) in mesothelioma has largely been restricted to determining that a lesion shows mesothelial differentiation, and genetic characterization of mesothelioma was an afterthought. This has changed dramatically over the past decade. While IHC still plays a major role in establishing mesothelial lineage, IHC can also be utilized in the differentiation of malignant from benign mesothelial proliferations. These advances are the direct result of an increased understanding of genetics that underlie mesothelial oncogenesis. This narrative review aims to summarize the most up-to-date data on the morphologic considerations in diagnosing mesothelioma, the use of IHC in mesothelioma, how IHC relates to the current understanding of mesothelial genetics, and how these topics currently relate to prognosis and potential uses in clinical decision making.
Many of these advances have come from international multi-institutional collaborations that have resulted in the highly cited series of pathologic diagnostic guidelines endorsed by the International Mesothelioma Interest Group (IMIG) and authored by IMIG members and mesothelioma experts (8-10). An updated 4th revision of these guidelines is currently in preparation. We present this article in accordance with the Narrative Review reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-23-22/rc).
Methods
English language articles from the PubMed database were searched on multiple occasions between the dates of April 1st, 2023, and May 5th, 2023 (Table 1, Table S1). Data search terms included “mesothelioma morphology”, “mesothelioma nuclear grade”, “mesothelioma transitional pattern”, “mesothelioma prognostic features”, “immunohistochemistry mesothelioma”, “immunohistochemistry mesothelioma prognosis”, “BAP1 mesothelioma”, “MTAP mesothelioma”, “mesothelioma cytology”, “mesothelioma PD-L1 expression”, and “mesothelioma immunotherapy”. The narrative review was created from PubMed search results from the list of search terms, along with the authors’ personal knowledge of the literature. The result of this process is summarized in the review below along with selected references.
Table 1
| Items | Specification |
|---|---|
| Date of search | Between 1st April 2023 and 5th May 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | Free text searches only using the following search terms: “mesothelioma morphology”, “mesothelioma nuclear grade”, “mesothelioma transitional pattern”, “mesothelioma prognostic features”, “immunohistochemistry mesothelioma”, “immunohistochemistry mesothelioma prognosis”, “BAP1 mesothelioma”, “MTAP mesothelioma”, “mesothelioma cytology”, “mesothelioma PD-L1 expression”, and “mesothelioma immunotherapy” |
| Timeframe | No timeframe restrictions on data |
| Inclusion and exclusion criteria | Inclusion criteria: |
| • English language texts only | |
| • All peer-reviewed studies and article types were acceptable | |
| • Studies focused on pleural mesothelioma unless discussion pertained to peritoneal mesothelioma | |
| Exclusion criteria: | |
| • Non-English language text | |
| • Full text unavailable via https://pubmed.ncbi.nlm.nih.gov/ links | |
| • Non-peer reviewed studies | |
| Selection process | Appropriate articles were selected by one author (Schulte JJ) based on relevance to topic being discussed |
Morphologic considerations
Assessment of mesothelial morphology starts with subclassification of mesothelioma into three histologic subtypes, epithelioid, biphasic, and sarcomatoid. These histologic subtypes have long been known to correlate to prognosis, with epithelioid morphology associated with the best overall median survival, followed by biphasic, and then sarcomatoid (11-13). Beyond prognostication, this basic histologic subtyping of mesothelioma has proven useful in surgical management, as surgery shows no benefit in patients with sarcomatoid morphology, and many also consider this to be true for patients with biphasic morphology (14,15). While morphologic subtype plays into the decision to proceed to surgical intervention, it should be highlighted that the diagnosis and initial classification of mesothelioma is often made after microscopic examination of only a small sampling of the tumor obtained by percutaneous or video-assisted thoracoscopic surgery (VATS). In this setting, we, along with others, have demonstrated that biphasic morphology is often underrepresented in small biopsies, and that one can expect approximately 20% of epithelioid mesotheliomas to show biphasic morphology if resected or additional tumor sampling is undertaken (16-19). Biphasic or sarcomatoid morphology, if identified in a biopsy, is highly specific for non-epithelioid morphology and should be considered representative of the true histologic subtype of the tumor (16).
Some authors have shown that there may be a prognostic significance associated with the percentage of epithelioid morphology in biphasic mesothelioma, ranging from 50–80% epithelioid morphology, but currently there is not enough data in the published literature to draw any firm conclusions (13,20). If a clinician does wish to incorporate the percentage of epithelioid morphology of biphasic mesotheliomas into any future treatment algorithm or, for the purposes of enrollment into a clinical trial, it is important to note that only a fair degree of agreement exists between biopsies and resection specimens in the quantification of percent epithelioid morphology (16).
Recently, a new morphologic pattern, transitional, has emerged in the literature. Transitional mesothelioma is defined as a pattern in which the mesothelial cells have lost some epithelioid morphology (plump and elongated; not as round as typical epithelioid cells), but are not overtly sarcomatoid (they retain some cellular cohesion in the form of sheet-like growth that is more typical of an epithelioid morphology) (21). In a previous edition of the World Health Organization (WHO), given the cellular cohesion of the transitional cells, transitional was considered a pattern of epithelioid mesothelioma (21,22). Studies now show that transitional pattern is strongly associated with poor prognosis, similar to the prognosis reported for sarcomatoid mesothelioma (13,23,24). The similarities in prognosis between transitional pattern and sarcomatoid mesothelioma are likely a result of underlying genetic similarities (25). While expert consensus originally advocated for transitional mesothelioma to be a pattern that could be observed in both epithelioid and sarcomatoid mesothelioma (21), the most recent WHO classification places transitional mesothelioma into a cytologic feature of sarcomatoid mesothelioma (26,27). Given that transitional is now regarded as a cytologic feature of sarcomatoid mesothelioma, an epithelioid mesothelioma with a transitional component should be regarded as biphasic mesothelioma (26).
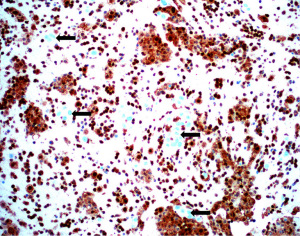
Beyond basic histologic subtyping and transitional features, numerous other architectural, cytologic, and stromal features have been found to be prognostically important in mesothelioma. A comprehensive review of all these features is beyond the scope of this text and has been outlined in other recently published works to which the reader if referred (10,21,27). Nevertheless, the reader should be made aware of one very powerful prognostic tool in epithelioid mesothelioma. This prognostic tool is the nuclear grading system. Nuclear grading in mesothelioma was originally developed by researchers at Memorial Sloan-Kettering Cancer Center in New York (28). This three-tier grading system (nuclear grades 1, 2, and 3), based on nuclear atypia and mitotic activity, was shown to stratify patients with epithelioid mesothelioma into three distinct prognostic groups; nuclear grade 1 (Figure 1) showed a median survival of 28 months, nuclear grade 2 (Figure 2) was 14 months, and nuclear grade 3 (Figure 3) was only 5 months. Subsequently, a large multicenter study by Rosen et al. reaffirmed these findings, but also showed that the presence of necrosis is an independent marker of adverse outcome (29). Rosen et al. also suggested that by combining the three-tier nuclear grade with the presence of necrosis, it is possible to further stratify these patients into distinct prognostic groupings (29). While never formally studied prior to adoption, expert consensus recommended the creation of a two-tier (high and low) nuclear grading system in which all nuclear grade 1 epithelioid mesotheliomas and nuclear grade 2 epithelioid mesotheliomas without necrosis are classified as low grade, while nuclear grade 2 epithelioid mesotheliomas with necrosis and all nuclear grade 3 epithelioid mesotheliomas are high grade (21,26). A later study showed that the two-tier grading system can be applied to biopsy specimens (30). The two-tier grading system is now recommended by the WHO to be reported on any specimen type and is a required synoptic reporting element in the College of American Pathologists’ (CAPs’) synoptic reporting for mesothelioma (27,31). Additional grading systems for mesothelioma have been proposed that incorporate other histopathologic parameters and can be applied to non-epithelioid subtypes of mesothelioma (32-34). Given the poor outcome typical of non-epithelioid mesotheliomas, it is the opinion of the authors and other experts that the clinical utility of such grading schemes is currently unclear and of limited value. There is only incomplete data that exists to suggest that histologic grading of epithelioid mesothelioma plays a role in treatment selection (35), and it is not yet routinely used in clinical decision-making guidelines. Nonetheless, it is obvious that nuclear grading remains one of the most powerful and robust prognostic tools generated by simple microscopic examination of hematoxylin and eosin (H&E) sections. With the widespread endorsement of nuclear grading by expert mesothelioma pathologists, the WHO, and CAP, it is anticipated that nuclear grading will someday be incorporated into clinical diagnostic and therapeutic algorithms.
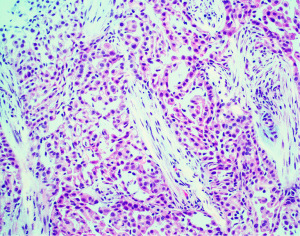
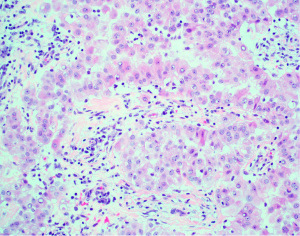
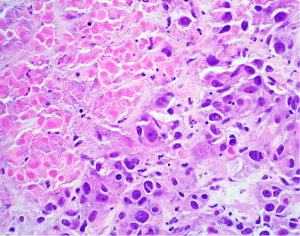
IHC in the diagnosis and classification of mesothelioma
IHC is an indispensable tool that is utilized by the pathologist in essentially every case of mesothelioma that is diagnosed. The principal use of IHC in mesothelial pathology is establishing mesothelial lineage. Published diagnostic guidelines support use of an IHC panel in the work-up of mesothelial lesions (10). The panel approach encourages using at least two mesothelial markers and two epithelial markers in the panel at a minimum (10). For example, an epithelioid pleural based lesion that is positive for two mesothelial markers and negative for two epithelial markers, would be supportive of a diagnosis of mesothelioma. The most commonly used antibodies that support mesothelial lineage include calretinin (Figure 4), WT-1 (Figure 5), and D2-40 (Figure 6), while common epithelial markers include Ber-EP4, MOC31, and carcinoembryonic antigen (CEA) (10,36-38). It is often useful, and necessary, to tailor the specific panel of stains to the differential being considered. For example, CK5/6 stains the majority of mesotheliomas, but also stains nearly all squamous cell carcinomas, so this marker is not useful if the two top diagnostic consideration are mesothelioma and pulmonary squamous cell carcinoma. Similarly, if one wishes to exclude metastatic ovarian carcinoma to the pleura prior to making a diagnosis of mesothelioma, WT-1 is a useless marker given the high degree of expression observed in both these tumors. The individual sensitivities and specificities of these markers and which stains perform best in each diagnostic scenario has been reviewed elsewhere and the reader is referred to these articles for further information (10,36).
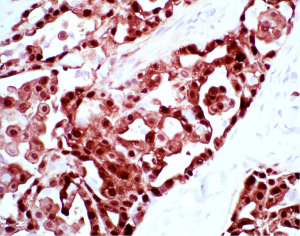
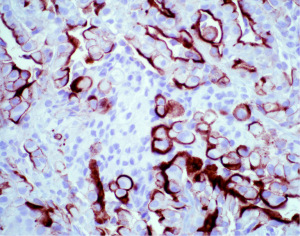
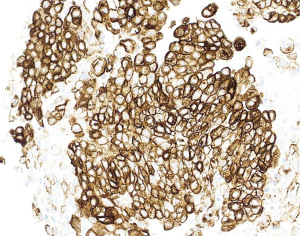
While many of the antibodies used in the workup of mesothelial lesions have been in use for years, more recently, a new mesothelial marker, HEG1 (Figure 7), and a new epithelial marker, claudin 4 (Figure 8), have come into the spotlight. HEG1 is a highly sensitive marker for epithelioid mesothelioma, with more limited use in biphasic and sarcomatoid mesothelioma (39-42). While HEG1 has great utility in the differential of lung adenocarcinoma versus mesothelioma, its use is diminished if ovarian serous carcinoma is being considered as there is considerable expression of HEG1 in ovarian epithelial tumors. Claudin 4 is the newest epithelial marker in use and it shows superior performance compared to more traditional epithelial markers (Ber-EP4 and MOC31), with some authors advocating that claudin 4 may be sufficient as a stand-alone IHC marker to exclude epithelial differentiation (43-45). The superiority of claudin 4 as a marker of epithelial lineage cannot be overstated. The literature has shown that older epithelial markers can often be expressed in mesothelial cells (up to 35% of cases) (45,46). While claudin 4 is an excellent marker for epithelial lineage, decreased to absent expression has been shown in poorly differentiated and sarcomatoid carcinomas (45,47-49). Given the strong performance of claudin 4 and with the excitement surrounding HEG1 as a new mesothelial marker, some authors have proposed a more limited panel of IHC stains (claudin 4 and HEG1 alone), but until HEG1 is more broadly studied and put into clinical practice, it is difficult at present to recommend deviation from the standard two mesothelial and two epithelial marker panel approach to mesothelioma diagnosis.
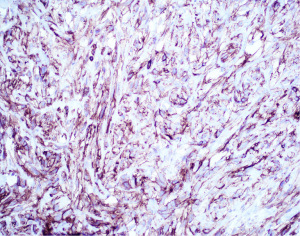
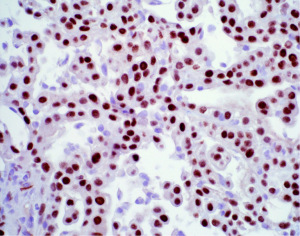
The presence of cytologic atypia and mitotic activity alone cannot be used to determine if a mesothelial proliferation is benign or malignant, and the gold standard for determining malignancy has historically relied on invasion of mesothelial cells into underlying tissue (chest wall, fibroadipose tissue, lung, etc.) (10). After establishing mesothelial lineage in a cellular proliferation, it may be difficult to diagnosis the lesion as malignant, especially in small biopsy specimens where underlying tissue may not be sampled. Advancements in the understanding of the genetic underpinnings of mesothelioma have been exploited to aid in the immunohistochemical distinction of benign and malignant mesothelial proliferations. Mutation in BAP1 is a frequent event in the pathogenesis of both sporadic and germline mutated mesothelioma (50-52). Nuclear loss of BAP1 expression by IHC (Figure 9) has emerged as a highly specific marker of malignancy in mesothelial proliferations. Loss of nuclear expression of BAP1 indicates a malignant mesothelial proliferation (53-58). While it may be tempting to include BAP1 nuclear loss as evidence of mesothelial differentiation, it should be noted that BAP1 loss can be seen in tumors other than mesothelioma (59). It is still recommended that other mesothelial markers are checked to ensure mesothelial differentiation. Nuclear loss of BAP1, while specific for malignancy, does not have high sensitivity (50–65% across all mesothelioma subtypes) (36). Loss of expression of BAP1 is most sensitive in epithelioid mesothelioma, reaching nearly 80% of cases, with notable drop-offs in sensitivity in non-epithelioid mesothelioma (56). Another common genetic event in mesothelioma is homozygous deletion of CDKN2A. Homozygous deletion of CDKN2A can be detected by fluorescence in situ hybridization (FISH) and is highly specific for malignancy in mesothelial proliferations (57,60,61). FISH technology is not as accessible or widely available as IHC. Fortunately, methylthioadenosine phosphorylase (MTAP), which sits adjacent to CDKN2A on chromosome 9 is frequently co-deleted with CDKN2A and an IHC stain for MTAP shows a high degree of correlation with CDKN2A homozygous deletion (57,60,62,63). In contrast to BAP1 in which nuclear loss of expression indicates malignancy in mesothelial proliferation, cytoplasmic loss of MTAP expression (Figure 10) is indicative of malignancy. As with BAP1, it is wise to first ensure that mesothelial lineage has been established with positive mesothelial markers, as MTAP can be lost in a large number of sarcomatoid carcinomas (64). Combining BAP1 and MTAP IHC together can increase the sensitivity for detecting malignancy in mesothelial proliferations (55,57,60,61). BAP1 and MTAP are robust IHC markers, but given imperfect sensitivity in detecting malignancy, some authors have proposed additional combinations of ancillary tests in further attempts to increase sensitivity, including use of p53, merlin, and YAP/TAZ IHC (55,65,66). As these various panels continue to be explored, hopefully a more refined panel will emerge with excellent sensitivity and specificity for malignancy in mesothelial proliferations.
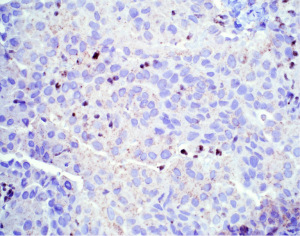
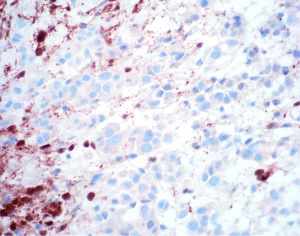
The emergence of BAP1 and MTAP IHC and CDKN2A FISH, has allowed for the identification of malignant mesothelial proliferations prior to invasion, termed mesothelioma in situ (Figure 11) (26,67-69). Mesothelioma in situ has long been proposed as a step in oncogenesis, but could not be proven until the development of these ancillary markers. Mesothelioma in situ is now an entity in the most recent WHO (27). Very few cases have thus far been collected. What is currently understood is that essentially all patients with mesothelioma in situ will go on to have progressive, eventually invasive disease. The time between detection of mesothelioma in situ and invasive disease ranges from months to years. An obvious question arises as how to best care for these patients. At present, there is no consensus on treatment recommendations given the novelty and rarity of the diagnosis. Now that pathologists are able to detect mesothermal in situ and it is an entity in the WHO, hopefully more cases and series will be reported in the literature and advancements in this space can be made.
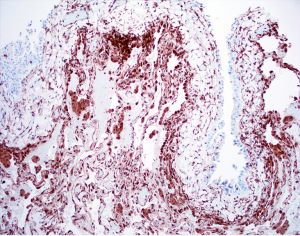
Beyond surgical pathology, IHC has also been employed in the classification of serous effusions (70-72). Of note, mesothelial and epithelial markers show excellence performance in pleural fluids, drawing particular attention again to the durability of claudin 4 in identifying epithelial lesions (73). The most interesting application of IHC in serosal fluids has been in the area of BAP1 and MTAP expression (Figures 12,13). As in surgical pathology, BAP1 and MTAP IHC have been shown to be able to discriminate benign and malignant mesothelial proliferations with nearly 100% specificity as shown by a recent meta-analysis (71). These IHC stains along with the concept of mesothelioma in situ are likely to bring about significant changes in the practice of serous fluid cytology and the reader is referred elsewhere for a more in-depth discussion (74-76).
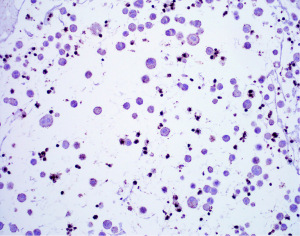
While beyond the scope of this review, it is also important to briefly mention the emerging diagnostic revolution underway brought about by digital pathology and artificial intelligence. These emerging technologies have shown various abilities ranging from basic histologic classification of tumors to prediction of underlying genetic alterations in malignancies (77-80). One important application of these technologies is in cytopathology. Cytology specimens may represent the only specimen for several patients with malignant pleural effusions. While the application of digital pathology and whole slide imaging (WSI) lags behind what has been done in surgical pathology, WSI can be employed by cytologists for accurate diagnosis and classification of cytologic preparations (81). Mobile devices may also be of utility in examination of cytology specimens which may improve diagnosis in underserved areas (82). Some work using artificial intelligence has been done specifically in mesothelioma using WSI, and one of these studies demonstrated that use of a deep-learning classification of mesothelioma resulted in improved prediction of patient outcome (24,83). Another recent study highlighted the power of deep learning to identify the transitional pattern in mesothelioma (25). These are exciting developments, yet the technology and its application to mesothelioma diagnosis and classification is still largely in its nascent form. Additional exciting developments are sure to arise in the coming years.
IHC and molecular testing as prognostic markers and in patient management
Some immunohistochemical markers have been shown to be of prognostic importance which in the future may play into treatment decisions. Loss of nuclear BAP1 staining is likely a favorable prognostic marker, especially when viewed in the context of germline mutations (56,58,84,85). Our group recently attempted to discern if the improved survival in patients with BAP1 mutation was a function of favorable histologic features being more common in BAP1 mutated mesotheliomas (86) We found that pleural epithelioid mesotheliomas that harbor BAP1 mutation were more likely to show low nuclear grade than those without BAP1 mutation, or with other mutations; this finding was not statistically significant when looking at peritoneal mesotheliomas alone. One study looking specifically at peritoneal mesothelioma did not show improved survival with loss of BAP1 IHC staining (87). Others have called into question the prognostic ability of BAP1 IHC especially when comparing BAP1 expression across the different histologic subtypes of mesothelioma (88). In contrast to the trend towards a better prognosis seen with BAP1 mutation, CDKN2A homozygous deletion and loss of cytoplasmic MTAP expression by IHC has been shown to be associated with a worse prognosis (87,89-91). While BAP1 and MTAP are the best studied IHC markers relating to prognostication, additional smaller studies have looked at various IHC markers including NF2, p16, and mesothelin, among others (87,89,91-95). If and how these IHC/molecular signatures may be incorporated into treatment approaches is unclear at present. Given what is currently understood of these markers and their likely prognostic ability, it is perfectly feasible to predict that some marker statuses may be used for enrollment into different arms of clinical trials in the future.
One of the more exciting treatment advancements in recent years has been the application of immunotherapy to mesothelioma with notable survival benefits being shown in non-epithelioid mesothelioma (96-98). It should be noted, nonetheless, that immunotherapy in mesothelioma is still in its infancy with only around 5% of patients receiving treatment (2). In the setting of BAP1 mutation, mesothelioma typically takes on an inflammatory immunophenotype, and while this is exciting in the setting of immunotherapy, the more recent data suggests a stronger survival advantage in non-epithelioid mesotheliomas even though BAP1 mutations are more common with epithelioid morphology (97,99). Mesotheliomas have also been shown to have high expression of VISTA, a gene associated with immune-checkpoint (100,101). Programmed death ligand-1 (PD-L1) expression has been tested in mesotheliomas and is more commonly associated with non-epithelioid morphology and a worse outcome (102-104). Mesothelioma expression of PD-L1, particularly in the peritoneum, can change over time following prior treatment; what this means for selecting patients for various treatment regimens is unclear at present (105). Neither routine staining nor interpretive guidelines for PD-L1 have been endorsed by any expert panel or clinical guideline group.
Key practical points on mesothelioma morphologic considerations
The above sections highlight the importance of accurate morphologic classification of mesothelioma. Unfortunately, achieving an accurate diagnosis and classification is often challenging. In this section, the authors herein offer some practical points to consider when rendering a diagnosis of mesothelioma.
Proving mesothelial lineage
The authors strongly advocate for continuing the practice of an IHC panel approach to establishing mesothelioma lineage (Table 2). Pathologists should be aware of the lack of specificity of the so called “mesothelioma markers”. Many of the markers of mesothelioma lineage stain carcinomas. Pathologists also need to be cognizant of the fact that mesotheliomas may occasionally show reactivity for classic pan-epithelial markers like Ber-EP4 and MOC31. In their everyday practice, the authors frequently rely on Claudin 4 as the most specific epithelial marker in excluding carcinoma. Pathologists should move to adopt this marker in their own laboratories or order the IHC using an external reference lab when working on a case in which the differential is carcinoma versus mesothelioma.
Table 2
| Carcinoma markers | Mesothelioma markers |
|---|---|
| Pan-epithelial markers | Calretinin |
| Claudin 4 | WT-1 |
| MOC31 | D2-40 |
| Ber-EP4 | CK5/6 |
| Other markers | HEG1 |
| TTF-1 | |
| p40 | |
| PAX8 | |
| Other carcinoma lineage specific markers depending on differential diagnosis |
Panel approach to mesothelioma diagnosis: use at least 2 mesothelioma markers and 2 carcinoma markers. IHC, immunohistochemistry.
Benign versus malignant mesothelial proliferations
Separating benign from malignant mesothelial proliferations remains a very challenging task. This is particularly true when dealing with fibrous pleuritis versus mesothelioma. A general rule of thumb is that fibrous pleuritis and other reactive mesothelial proliferations show orderly and predictable arrangement of mesothelial cells. Mesotheliomas often show a full thickness proliferation of mesothelial cells that is often architecturally complex or haphazardly arranged. The morphologic differences between benign and malignant mesothelial proliferation have been well described elsewhere. The authors feel that a more recent challenge has not been the separation of a fibrous pleuritis from mesothelioma, but rather the separation of benign hyperplasia and other tumors and proliferations (well differentiated papillary mesothelial tumor, etc.) from mesothelioma. In this case, the authors strongly advocate for the use of the markers of malignancy (BAP1, MTAP, CDKN2A FISH; Table 3). While these markers are not in widespread use in every laboratory, at least within North America, they are easily orderable through large reference laboratories or academic medical centers. One final note on the morphologic classification of mesothelioma is that the pathologist should not forget about the diagnostic power harbored within a simple cytokeratin IHC. Pankeratins, CK5/6, and CK7 highlight many mesotheliomas. While this is a well-known fact, careful review of cytokeratin staining may reveal very useful information. Cytokeratin may show subtle invasion of individual tumor cells into underlying tissue, proving a mesothelial proliferation is malignant. Cytokeratin may also highlight architectural patterns in a way that may not be obvious on H&E-stained sections. Cytokeratins may show epithelial islands in an otherwise sarcomatoid mesothelioma, allowing for reclassification as biphasic mesothelioma. Also, cytokeratin may highlight subtle spindling of tumor cells in an epithelioid mesothelioma, cluing in the pathologist of the possible presence of transitional or sarcomatoid morphologies.
Table 3
| Step | ||
|---|---|---|
| Step 1 | Establish mesothelial lineage | See Table 2 |
| Step 2 | Identify invasion into underlying tissue | Diagnostic of malignancy if present |
| Step 3 | If no invasion: IHC stains BAP1 or MTAP | Nuclear loss BAP1 = malignant |
| Cytoplasmic loss MTAP = malignant | ||
| Step 4 | BAP1 and MTAP retained—perform CDKN2A FISH | Deletion of CDKN2A = malignant |
| Step 5 | If no deletion of CDKN2A: diagnose “atypical mesothelial proliferation” or go to step 6 | Recommend additional tissue sampling |
| Step 6 | Perform molecular sequencing (if available) | Identification of mutations commonly seen in mesothelioma is supportive of malignancy |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization.
Diagnosis of mesothelioma in cytology
Recently it has become possible to establish malignancy in mesothelial proliferations on review of serous effusions. The authors have advocated in their own clinical practice, the adoption of BAP1 and MTAP IHC for use in cytology specimens, especially when an adequate cell block is made. Nuclear loss of BAP1 and cytoplasmic loss of MTAP in mesothelial cells is always indicative of a malignant mesothelial proliferation. This is true in both surgical pathology and cytopathology. The one important caveat to the cytologic diagnosis of mesothelioma, is that it is currently unknown how well the cell block is predictive of final subtype. In the limited number of cases in which the authors have helped to establish a diagnosis of mesothelioma on cell block (all of them showing epithelioid morphology), they have advocated for pleural biopsy to subtype the mesothelioma. In a number of these cases, a sarcomatoid component was found in the pleural biopsy, changing the tumor classification from epithelioid to biphasic.
Conclusions
The diagnosis and classification of mesothelioma has moved from its earlier simplistic forms based purely on histologic subtyping. Numerous IHC markers exist which are able to distinguish benign and malignant mesothelial proliferations, even prior to invasion and in effusion cytology specimens. Some of these markers, most notably BAP1 and MTAP, allow for identification of mesothelioma in situ and function as disease prognosticators. With the advent of immunotherapy in mesothelioma, there is the potential to significantly alter disease course, and thus far there are some promising results for non-epithelioid mesotheliomas. So long as these immunophenotypic and molecular advancements in mesotheliomas continue to populate the literature, there will be an enduring hope that these markers can help guide patients to appropriate and more effective therapies. There will no doubt be much to come on this topic in the next few years.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wickii T. Vigneswaran) for the series “Malignant Pleural Mesothelioma” published in Shanghai Chest. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-23-22/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-23-22/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-23-22/coif). The series “Malignant Pleural Mesothelioma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003-2008. Int J Occup Environ Health 2013;19:1-10. [Crossref] [PubMed]
- Bou-Samra P, Chang A, Azari F, et al. Epidemiological, therapeutic, and survival trends in malignant pleural mesothelioma: A review of the National Cancer Database. Cancer Med 2023;12:12208-20. [Crossref] [PubMed]
- Zhao J, Zuo T, Zheng R, et al. Epidemiology and trend analysis on malignant mesothelioma in China. Chin J Cancer Res 2017;29:361-8. [Crossref] [PubMed]
- Ceruti P, Lonni S, Baglivo F, et al. Endoscopic diagnosis and management of pleural effusion in malignant pleural mesothelioma. J Thorac Dis 2018;10:S269-75. [Crossref] [PubMed]
- Xu LL, Yang Y, Wang Z, et al. Malignant pleural mesothelioma: diagnostic value of medical thoracoscopy and long-term prognostic analysis. BMC Pulm Med 2018;18:56. [Crossref] [PubMed]
- Shaikh F, Lentz RJ, Feller-Kopman D, et al. Medical thoracoscopy in the diagnosis of pleural disease: a guide for the clinician. Expert Rev Respir Med 2020;14:987-1000. [Crossref] [PubMed]
- Scherpereel A, Opitz I, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020;55:1900953. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133:1317-31. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordóñez NGGuidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group, et al. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015;196:23-32. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Galateau Salle F, Le Stang N, Nicholson AG, et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J Thorac Oncol 2018;13:1189-203. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Woodard GA, Jablons DM. Surgery for pleural mesothelioma, when it is indicated and why: arguments against surgery for malignant pleural mesothelioma. Transl Lung Cancer Res 2020;9:S86-91. [Crossref] [PubMed]
- Schulte JJ, Chapel DB, Attanoos R, et al. Comparison of Nuclear Grade, Necrosis, and Histologic Subtype Between Biopsy and Resection in Pleural Malignant Mesothelioma: An International Multi-Institutional Analysis. Am J Clin Pathol 2021;156:989-99. [Crossref] [PubMed]
- Bueno R, Reblando J, Glickman J, et al. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg 2004;78:1774-6. [Crossref] [PubMed]
- Kao SC, Yan TD, Lee K, et al. Accuracy of diagnostic biopsy for the histological subtype of malignant pleural mesothelioma. J Thorac Oncol 2011;6:602-5. [Crossref] [PubMed]
- Chirieac LR, Hung YP, Foo WC, et al. Diagnostic value of biopsy sampling in predicting histology in patients with diffuse malignant pleural mesothelioma. Cancer 2019;125:4164-71. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of Epithelioid Differentiation Is a Predictor of Survival in Malignant Pleural Mesothelioma. Ann Thorac Surg 2017;103:962-6. [Crossref] [PubMed]
- Nicholson AG, Sauter JL, Nowak AK, et al. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J Thorac Oncol 2020;15:29-49. [Crossref] [PubMed]
- WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer; 2015.
- Dacic S, Le Stang N, Husain A, et al. Interobserver variation in the assessment of the sarcomatoid and transitional components in biphasic mesotheliomas. Mod Pathol 2020;33:255-62. [Crossref] [PubMed]
- Courtiol P, Maussion C, Moarii M, et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med 2019;25:1519-25. [Crossref] [PubMed]
- Galateau Salle F, Le Stang N, Tirode F, et al. Comprehensive Molecular and Pathologic Evaluation of Transitional Mesothelioma Assisted by Deep Learning Approach: A Multi-Institutional Study of the International Mesothelioma Panel from the MESOPATH Reference Center. J Thorac Oncol 2020;15:1037-53. [Crossref] [PubMed]
- Sauter JL, Dacic S, Galateau-Salle F, et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J Thorac Oncol 2022;17:608-22. [Crossref] [PubMed]
- Thoracic Tumours. In: WHO Classification of Tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2021.
- Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71. [Crossref] [PubMed]
- Rosen LE, Karrison T, Ananthanarayanan V, et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod Pathol 2018;31:598-606. [Crossref] [PubMed]
- Zhang YZ, Brambilla C, Molyneaux PL, et al. Presence of pleomorphic features but not growth patterns improves prognostic stratification of epithelioid malignant pleural mesothelioma by 2-tier nuclear grade. Histopathology 2020;77:423-36. [Crossref] [PubMed]
- Schneider F, Roden R, Dacic S, et al. Protocol for the Examination of Specimens From Patients With Malignant Pleural Mesothelioma. 2021. Available online: https://documents.cap.org/protocols/PleuraPericard_4.1.0.0.REL_CAPCP.pdf
- Pelosi G, Papotti M, Righi L, et al. Pathologic Grading of Malignant Pleural Mesothelioma: An Evidence-Based Proposal. J Thorac Oncol 2018;13:1750-61. [Crossref] [PubMed]
- Fuchs TL, Chou A, Aksoy Y, et al. A Critical Assessment of Current Grading Schemes for Diffuse Pleural Mesothelioma With a Proposal for a Novel Mesothelioma Weighted Grading Scheme (MWGS). Am J Surg Pathol 2022;46:774-85. [Crossref] [PubMed]
- Aksoy Y, Chou A, Mahjoub M, et al. A novel prognostic nomogram for predicting survival in diffuse pleural mesothelioma. Pathology 2023;55:449-55. [Crossref] [PubMed]
- Türk İ, Findik G, Çetin M, et al. Importance of Histopathological Grading for Treatment Selection in Malignant Mesothelioma. Thorac Cardiovasc Surg 2023;71:497-503. [Crossref] [PubMed]
- Chapel DB, Schulte JJ, Husain AN, et al. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl Lung Cancer Res 2020;9:S3-S27. [Crossref] [PubMed]
- Yaziji H, Battifora H, Barry TS, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 2006;19:514-23. [Crossref] [PubMed]
- Ordóñez NG. Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol 2013;44:1-19. [Crossref] [PubMed]
- Hiroshima K, Wu D, Koh E, et al. Membranous HEG1 expression is a useful marker in the differential diagnosis of epithelioid and biphasic malignant mesothelioma versus carcinomas. Pathol Int 2021;71:604-13. [Crossref] [PubMed]
- Naso JR, Tsuji S, Churg A. HEG1 Is a Highly Specific and Sensitive Marker of Epithelioid Malignant Mesothelioma. Am J Surg Pathol 2020;44:1143-8. [Crossref] [PubMed]
- Tsuji S, Washimi K, Kageyama T, et al. HEG1 is a novel mucin-like membrane protein that serves as a diagnostic and therapeutic target for malignant mesothelioma. Sci Rep 2017;7:45768. [Crossref] [PubMed]
- Churg A, Naso JR. Hypothesis: HEG1 and claudin-4 staining will allow a diagnosis of epithelioid and biphasic mesothelioma versus non-small-cell lung carcinoma with only two stains in most cases. Histopathology 2023;82:385-92. [Crossref] [PubMed]
- Najjar S, Gan Q, Stewart J, et al. The utility of claudin-4 versus MOC-31 and Ber-EP4 in the diagnosis of metastatic carcinoma in cytology specimens. Cancer Cytopathol 2023;131:245-53. [Crossref] [PubMed]
- Elhosainy A, Hafez MMA, Yassin EH, et al. Diagnostic Value of Claudin-4 and EZH2 Immunohistochemistry in Effusion Cytology. Asian Pac J Cancer Prev 2022;23:2779-85. [Crossref] [PubMed]
- Naso JR, Churg A. Claudin-4 shows superior specificity for mesothelioma vs non-small-cell lung carcinoma compared with MOC-31 and Ber-EP4. Hum Pathol 2020;100:10-4. [Crossref] [PubMed]
- Zhu Y, Moore S, Wang A, et al. Comprehensive epithelial biomarker analysis of malignant mesothelioma: EpCAM positivity is a potential diagnostic pitfall. Cancer Cytopathol 2023;131:507-15. [Crossref] [PubMed]
- Zuccatosta L, Bizzarro T, Rossi G, et al. Immunohistochemistry for Claudin-4 and BAP1 in the Differential Diagnosis between Sarcomatoid Carcinoma and Sarcomatoid Mesothelioma. Diagnostics (Basel) 2023;13:249. [Crossref] [PubMed]
- Ohta Y, Sasaki Y, Saito M, et al. Claudin-4 as a marker for distinguishing malignant mesothelioma from lung carcinoma and serous adenocarcinoma. Int J Surg Pathol 2013;21:493-501. [Crossref] [PubMed]
- Lee SK, Moon J, Park SW, et al. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep 2005;13:193-9. [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Panou V, Gadiraju M, Wolin A, et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol 2018;36:2863-71. [Crossref] [PubMed]
- Leblay N, Leprêtre F, Le Stang N, et al. BAP1 Is Altered by Copy Number Loss, Mutation, and/or Loss of Protein Expression in More Than 70% of Malignant Peritoneal Mesotheliomas. J Thorac Oncol 2017;12:724-33. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol 2016;40:714-8. [Crossref] [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- Chapel DB, Hornick JL, Barlow J, et al. Clinical and molecular validation of BAP1, MTAP, P53, and Merlin immunohistochemistry in diagnosis of pleural mesothelioma. Mod Pathol 2022;35:1383-97. [Crossref] [PubMed]
- McGregor SM, Dunning R, Hyjek E, et al. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670-8. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: Comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 2017;104:98-105. [Crossref] [PubMed]
- Righi L, Duregon E, Vatrano S, et al. BRCA1-Associated Protein 1 (BAP1) Immunohistochemical Expression as a Diagnostic Tool in Malignant Pleural Mesothelioma Classification: A Large Retrospective Study. J Thorac Oncol 2016;11:2006-17. [Crossref] [PubMed]
- Kwon J, Lee D, Lee SA. BAP1 as a guardian of genome stability: implications in human cancer. Exp Mol Med 2023;55:745-54. [Crossref] [PubMed]
- Berg KB, Dacic S, Miller C, et al. Utility of Methylthioadenosine Phosphorylase Compared With BAP1 Immunohistochemistry, and CDKN2A and NF2 Fluorescence In Situ Hybridization in Separating Reactive Mesothelial Proliferations From Epithelioid Malignant Mesotheliomas. Arch Pathol Lab Med 2018;142:1549-53. [Crossref] [PubMed]
- Kinoshita Y, Hida T, Hamasaki M, et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol 2018;126:54-63. [Crossref] [PubMed]
- Hamasaki M, Kinoshita Y, Yoshimura M, et al. Cytoplasmic MTAP expression loss detected by immunohistochemistry correlates with 9p21 homozygous deletion detected by FISH in pleural effusion cytology of mesothelioma. Histopathology 2019;75:153-5. [Crossref] [PubMed]
- Chapel DB, Schulte JJ, Berg K, et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 2020;33:245-54. [Crossref] [PubMed]
- Terra S, Roden AC, Yi ES, et al. Loss of Methylthioadenosine Phosphorylase by Immunohistochemistry Is Common in Pulmonary Sarcomatoid Carcinoma and Sarcomatoid Mesothelioma. Am J Clin Pathol 2022;157:33-9. [Crossref] [PubMed]
- Li Y, Yang SR, Chen YB, et al. Neurofibromatosis Type 2-Yes-Associated Protein and Transcriptional Coactivator With PDZ-Binding Motif Dual Immunohistochemistry Is a Reliable Marker for the Detection of Neurofibromatosis Type 2 Alterations in Diffuse Pleural Mesothelioma. Mod Pathol 2023;36:100030. [Crossref] [PubMed]
- Naso JR, Tessier-Cloutier B, Senz J, et al. Significance of p53 immunostaining in mesothelial proliferations and correlation with TP53 mutation status. Mod Pathol 2022;35:77-81. [Crossref] [PubMed]
- Churg A, Hwang H, Tan L, et al. Malignant mesothelioma in situ. Histopathology 2018;72:1033-8. [Crossref] [PubMed]
- Churg A, Dacic S, Galateau-Salle F, et al. Malignant Mesothelioma In Situ: Clinical and Pathologic Implications. J Thorac Oncol 2020;15:899-901. [Crossref] [PubMed]
- Churg A, Galateau-Salle F, Roden AC, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol 2020;33:297-302. [Crossref] [PubMed]
- Shaker N, Wu D, Abid AM. Cytology of malignant pleural mesothelioma: Diagnostic criteria, WHO classification updates, and immunohistochemical staining markers diagnostic value. Diagn Cytopathol 2022;50:532-7. [Crossref] [PubMed]
- Girolami I, Lucenteforte E, Eccher A, et al. Evidence-based diagnostic performance of novel biomarkers for the diagnosis of malignant mesothelioma in effusion cytology. Cancer Cytopathol 2022;130:96-109. [Crossref] [PubMed]
- Monaco SE, Brcic L, Dacic S. State-of-the-art cytology of pleural fluid, focusing on the diagnosis of mesothelioma. Cytopathology 2022;33:57-64. [Crossref] [PubMed]
- Vojtek M, Walsh MD, Papadimos DJ, et al. Claudin-4 immunohistochemistry is a useful pan-carcinoma marker for serous effusion specimens. Cytopathology 2019;30:614-9. [Crossref] [PubMed]
- Klebe S, Nakatani Y, Dobra K, et al. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology 2021;53:446-53. [Crossref] [PubMed]
- Klebe S, Galateau Salle F, Bruno R, et al. The highlights of the 15th international conference of the international mesothelioma interest group - Do molecular concepts challenge the traditional approach to pathological mesothelioma diagnosis? Lung Cancer 2022;163:1-6. [Crossref] [PubMed]
- Michael CW, Bedrossian CCWM, Sadri N, et al. The cytological features of effusions with mesothelioma in situ: A report of 9 cases. Diagn Cytopathol 2023;51:374-88. [Crossref] [PubMed]
- Fu Y, Jung AW, Torne RV, et al. Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis. Nat Cancer 2020;1:800-10. [Crossref] [PubMed]
- Heinz CN, Echle A, Foersch S, et al. The future of artificial intelligence in digital pathology - results of a survey across stakeholder groups. Histopathology 2022;80:1121-7. [Crossref] [PubMed]
- Kather JN, Heij LR, Grabsch HI, et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat Cancer 2020;1:789-99. [Crossref] [PubMed]
- Dolezal JM, Wolk R, Hieromnimon HM, et al. Deep learning generates synthetic cancer histology for explainability and education. NPJ Precis Oncol 2023;7:49. [Crossref] [PubMed]
- Eccher A, Girolami I. Current state of whole slide imaging use in cytopathology: Pros and pitfalls. Cytopathology 2020;31:372-8. [Crossref] [PubMed]
- Santonicco N, Marletta S, Pantanowitz L, et al. Impact of mobile devices on cancer diagnosis in cytology. Diagn Cytopathol 2022;50:34-45. [Crossref] [PubMed]
- Choudhury A. Predicting cancer using supervised machine learning: Mesothelioma. Technol Health Care 2021;29:45-58. [Crossref] [PubMed]
- Markowitz P, Patel M, Groisberg R, et al. Genomic characterization of malignant pleural mesothelioma and associated clinical outcomes. Cancer Treat Res Commun 2020;25:100232. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Chen-Yost HI, Tjota MY, Gao G, et al. Characterizing the distribution of alterations in mesothelioma and their correlation to morphology. Am J Clin Pathol 2023;160:238-46. [Crossref] [PubMed]
- Singhi AD, Krasinskas AM, Choudry HA, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol 2016;29:14-24. [Crossref] [PubMed]
- Cantini L, Pecci F, Murrone A, et al. Questioning the prognostic role of BAP-1 immunohistochemistry in malignant pleural mesothelioma: A single center experience with systematic review and meta-analysis. Lung Cancer 2020;146:318-26. [Crossref] [PubMed]
- Jean D, Daubriac J, Le Pimpec-Barthes F, et al. Molecular changes in mesothelioma with an impact on prognosis and treatment. Arch Pathol Lab Med 2012;136:277-93. [Crossref] [PubMed]
- Ma GY, Shi S, Wang P, et al. Clinical significance of 9P21 gene combined with BAP1 and MTAP protein expression in diagnosis and prognosis of mesothelioma serous effusion. Biomed Rep 2022;17:66. [Crossref] [PubMed]
- Brcic L, Le Stang N, Gallob F, et al. A Combination of MTAP and p16 Immunohistochemistry Can Substitute for CDKN2A Fluorescence In Situ Hybridization in Diagnosis and Prognosis of Pleural Mesotheliomas. Arch Pathol Lab Med 2023;147:313-22. [Crossref] [PubMed]
- Forest F, Patoir A, Dal Col P, et al. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: prognostic implications. Pathology 2018;50:635-41. [Crossref] [PubMed]
- Chou A, Toon CW, Clarkson A, et al. The epithelioid BAP1-negative and p16-positive phenotype predicts prolonged survival in pleural mesothelioma. Histopathology 2018;72:509-15. [Crossref] [PubMed]
- Wang L, Wang X, Sun N, et al. Correlation of serum-soluble mesothelin-related protein, HMGB1 and CA125 in diffuse malignant peritoneal mesothelioma and their combined measurement for prognosis. Scand J Clin Lab Invest 2023;83:74-8. [Crossref] [PubMed]
- Chu GJ, Linton A, Kao S, et al. High mesothelin expression by immunohistochemistry predicts improved survival in pleural mesothelioma. Histopathology 2023;83:202-10. [Crossref] [PubMed]
- Mielgo-Rubio X, Cardeña Gutiérrez A, Sotelo Peña V, et al. Tsunami of immunotherapy reaches mesothelioma. World J Clin Oncol 2022;13:267-75. [Crossref] [PubMed]
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 2022;33:488-99. [Crossref] [PubMed]
- Ladanyi M, Sanchez Vega F, Zauderer M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med 2019;11:18. [Crossref] [PubMed]
- Hmeljak J, Sanchez-Vega F, Hoadley KA, et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov 2018;8:1548-65. [Crossref] [PubMed]
- Muller S, Victoria Lai W, Adusumilli PS, et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod Pathol 2020;33:303-11. [Crossref] [PubMed]
- Chapel DB, Stewart R, Furtado LV, et al. Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28-8 pharmDx assays. Hum Pathol 2019;87:11-7. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. [Crossref] [PubMed]
- Combaz-Lair C, Galateau-Sallé F, McLeer-Florin A, et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol 2016;52:9-18. [Crossref] [PubMed]
- White MG, Schulte JJ, Xue L, et al. Heterogeneity in PD-L1 expression in malignant peritoneal mesothelioma with systemic or intraperitoneal chemotherapy. Br J Cancer 2021;124:564-6. [Crossref] [PubMed]
Cite this article as: Schulte JJ, Husain AN. Morphology and immunohistochemical and molecular markers for diagnosis and guiding therapy in mesothelioma: a narrative review. Shanghai Chest 2023;7:31.


