Uniportal-video-assisted thoracoscopic surgery (VATS) resection of multiple left thoracic neurofibromas: a case report
Highlight box
Key findings
• Chest wall surgery is often surgical demanding and performed by highly invasive approaches. We report a case of a 40-year-old man affected by multiple thoracic neurofibromas for the first time resected by uniportal video-assisted thoracoscopic surgery (VATS), with good surgical outcomes.
What is known and what is new?
• Till now, only a few cases of thoracic neurofibromas have been described in literature due to the rarity of the pathology. Only one case was approached by minimally invasive triportal-VATS, and most of them were performed by different open accesses (thoracotomy, sternotomy);
• It’s the first case of thoracic neurofibromas approached by uniportal-VATS, that seems to be a promising technique for the treatment of chest-wall lesions, with good surgical outcomes.
What is the implication, and what should change now?
• Uniportal-VATS requires good skills in thoracoscopy, but in the hands of expert surgeons, it is a safe and feasible approach, as it seems to be also for chest wall lesions, allowing fast recovery to patient, low post-operative pain and good cosmetic results.
Introduction
Mediastinal and chest wall tumors represent a various spectrum of neoplasm. Differential diagnosis of these lesions may be challenging and could require a multidisciplinary approach in order to provide the best treatment and outcomes. Among chest-wall lesions, neurofibromatosis is one of the most frequent pathologies presenting thoracic masses (1). Chest wall surgery is often surgical demanding and performed by highly invasive approaches. Few cases are reported in literature managed by minimally invasive surgery [by triportal-video-assisted thoracic surgery (VATS) approach]. We report the first case of multiple thoracic neurofibromas resected by uniportal VATS, with good surgical outcomes. We present the following case in accordance with CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-22-59/rc).
Case presentation
A 40-year-old man with neurofibromatosis type 1 (NF1) (negative for NF-1 gene research) underwent several surgical treatments for multiple neurofibromas located in bilateral supraclavicular region, lumbar spine, bilateral radial nerve, nasal mucosa and cerebellum-pontine angle. Patient’s family history was negative for neurofibromatosis and multiple NF-1 gene examinations resulted negative. In 2001, he also underwent right side hemicolectomy for cell B lymphoma. No other pathologies were described in anamnesis. During clinic-radiological follow-up at rare neoplasm day-hospital of our center, computed tomography (CT) scans documented a slight dimensional increase (about 8–10 mm on major axes since first CT of 2014) of two left endothoracic well-defined masses.
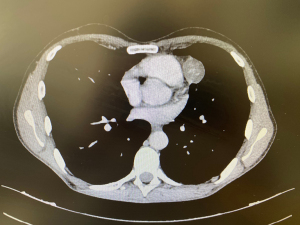
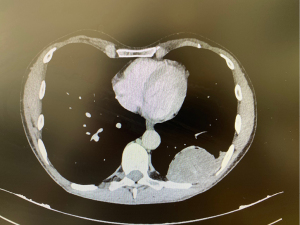
Last CT scan of October 2022 showed the following dimensions: the lesion located in pericardial region, along the phrenic nerve, was about 31 mm (on max diameter) (Figure 1), the second lesion, tightly adhering on the posterior arch of the 9th rib, and presenting radiological scalloping phenomenon, was about 70 mm × 60 mm (Figure 2).
In consideration of the basic pathology and of the slight dimension increase of lesions during the time, associated to left chest pain onset and neuralgia since months, the patient was scheduled for surgical treatment after multidisciplinary discussion. Uniportal-VATS was the approach chouse, as for all thoracic procedures at our center since 2016 (2).
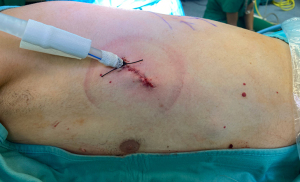
A single 4-cm incision on the 5th intercostal space was performed on middle axillary line, with patient lying on right side, in single-lung ventilation) (Figure 3).
A wound protector (Alexis® Small) was placed to protect the intercostal space and for preventing soiling of the camera (30° 10 mm) during the procedure.
The first mass was located along the phrenic nerve and tightly adhered to it and to the pericardium. An accurate blunt dissection was performed by energy-device (LigaSureTM Maryland Medtronic), obtaining a complete resection with phrenic nerve sparing. For improving the exposure of the lesion and improving its dissection, the operative table was tilted towards the back side of the patient with both operators standing on his ventral position (and the camera assistant on caudal part of the patient).
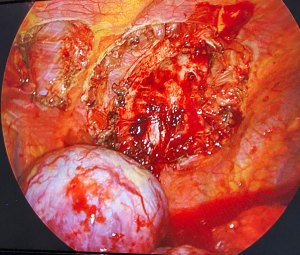
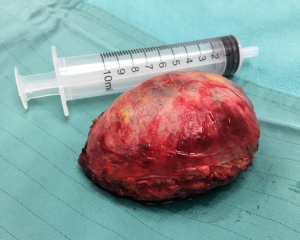
The second lesion appeared to be strictly adhered to the posterior arch of the 9th rib, as previously confirmed at CT scan. For better approaching it, the table was tilted 40°–45° towards the surgeons. After accurate dissection of the lesion from rib surface, we confirmed that the periosteum and intercostal muscle were not infiltrated but only imprinted, as in Figure 4. Thus, costal resection was not required. Surgery duration was about 100 minutes. Blood loss was null. Both masses were extracted intact trough the incision using an endobag. At the end of surgery, only one 28-Fr chest tube was left in place, through the same intercostal incision. The resected tumors had an elastic and smooth consistency at touch (Figure 5). Histological evaluation confirmed both lesions as Schwannoma, with margins free from disease.
Post-operative course was uneventful, with chest pain relief immediately after surgery. Chest tube was removed on the second post-operative day, with normal chest X-ray at discharge.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office.
Discussion
Mediastinal and chest wall tumors include a variety of neoplasm; schwannoma and neurofibroma represent the most common mediastinal neurogenic tumors and rarely can degenerate into malignant tumours (3). These neurogenic mediastinal masses arise from nerve sheath and frequently are incidentally discovered, nevertheless for genetic mutational syndrome like neurofibromatosis whose manifestations can be even predictable (4).
Neurofibromatosis is a heterogenic group which includes three different diseases, NF1, neurofibromatosis type 2 (NF2) and schwannomatosis. NF1 is the most common of these three conditions and is one of the most frequently diagnosed cancer susceptibility disorders which involve the nervous system (5). Neurofibromatosis is a genetic disease of NF1 tumor suppressor gene which encodes a large protein (neurofibromin) that primarily works as a RAS protein negative regulator: this might suggest that targeted therapy for NF1 could derive from inhibition of the RAS signaling pathway (6).
Patient affected by NF1 can develop several manifestations such as pigmentary abnormalities (coffee with milk skin spots), peripheral nerve sheath tumors [neurofibromas and malignant peripheral nerve sheath tumors (MPNSTs)], astrocytomas (optic pathway gliomas), seizures, strokes, skeletal deformities (osteopenia, scoliosis, sphenoid wing dysplasia, congenital tibial dysplasia, and pseudarthrosis, macrocephaly) and vascular abnormalities (7). Neurofibromas are benign Schwann-cell tumors composed by neoplastic Schwann cells and by non-neoplastic fibroblasts, mast cells, macrophages, endothelial cells, pericytes, and perineural cells (8). Many research and advances in molecular biology and mouse models of disease did facilitate targeted therapy to provide a faster, indolent, and advance treatment (9). Clinical and radiological classification are the most important challenge for physicians. Therefor accurate diagnosis is important to individualize clinical care and further therapy. The mainstay of management is age specific monitoring of disease manifestations, that are most likely to become symptomatic in severe disease at all ages. (10). Clinical monitoring is essential and by the new guidelines, baseline brain, spine magnetic resonance imaging (MRI) and routine chests and abdomen imaging to identify asymptomatic tumors, do not influence management and should not be undertaken (10). Thoracic symptoms can variate related to dimension and can manifest with dyspnea, cough, chest pain, back pain. The surgical procedure for chest tumors is often challenging and highly invasive. These masses can also invade important anatomical structures into thoracic cavity such as costs, muscles and pericardial tissue, making surgical treatment complex; it is sometimes necessary to reconstruct thoracic wall after large resection to protect internal organs and to prevent abnormal pulmonary ventilation and herniation (11). The best methods to reconstruct thoracic wall after resection depend on the size of the tumor, depth and location in order to choose the best material and technique to rebuild (12). Therefore, a very accurate pre-operative study is mandatory.
Thoracoscopic approach for removal of this kind of lesions is always challenging, due to tumor size and possible chest wall infiltration, and requires experienced surgeons with good skills in minimally invasive surgery. Till now few cases of thoracic neurofibromas have been described in literature due to the rarity of the pathology, only one case was approached by triportal-VATS (13), and most of them were performed by different open accesses (thoracotomy, sternotomy) (14-16).
Compared to triportal-VATS, uniportal-VATS gives the theorical advantage of less post-operative pain and neuralgia, due to involvement of only an intercostal space (protected during procedure by a wound protector softer than standard trocars commonly used in triportal-VATS (17). This contributes to improve patient’s recovery after surgery and satisfaction. Furthermore, the Uniportal-access gives the surgeons the possibility to have a good exposure (of lesions either of anterior that posterior mediastinum), to introduce several instruments at the same time (as ring forceps for grasping the lesions, dissectors and energy-devices), with the same hand-eye coordination as in open surgery (18). Uniportal-VATS requires good skills in thoracoscopy and dexterity but, in the hands of expert surgeons, it is a safe and feasible approach (19), as it seems to be also for chest wall lesions, allowing fast recovery to patient, low post-operative pain and good cosmetic results (20).
Conclusions
To the best of our knowledge, our report is the first case of thoracic neurofibromas approached by uniportal-VATS, that seems to be a promising technique for the treatment of chest-wall lesions, with good surgical outcomes.
Minimally invasive approach in these cases can spare a heavier open surgical treatment, providing quick recovery, pain control and a better cosmetic result especially in young patients. Large series are advocated to confirm our results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-59/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-59/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marchevsky AM, Balzer B. Mediastinal tumors of peripheral nerve origin (so-called neurogenic tumors). Mediastinum 2020;4:32. [Crossref] [PubMed]
- Nachira D, Meacci E, Petracca Ciavarella L, et al. Uniportal video-assisted thoracic surgery Roman experience-a report of the first 16-month Roman experience. J Thorac Dis 2018;10:S3678-85. [Crossref] [PubMed]
- Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344-57. [Crossref] [PubMed]
- Issoufou I, Lakranbi M, Sani R, et al. Neurogenic mediastinal tumors in adults. Rev Pneumol Clin 2016;72:310-5. [Crossref] [PubMed]
- Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol 2018;148:799-811. [Crossref] [PubMed]
- Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin 2002;20:841-65. [Crossref] [PubMed]
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014;13:834-43. [Crossref] [PubMed]
- Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene 2007;26:4609-16. [Crossref] [PubMed]
- Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol 2013;115:939-55. [Crossref] [PubMed]
- Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet 2007;44:81-8. [Crossref] [PubMed]
- Dai Z, Maihemuti M, Sun Y, et al. Resection and reconstruction of huge tumors in the chest wall. J Cardiothorac Surg 2022;17:116. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- Azuma Y, Tochigi N, Sano A, et al. Thoracoscopic resection of bilateral multiple superior mediastinal neurofibromas. J Cardiothorac Surg 2021;16:310. [Crossref] [PubMed]
- Kanzaki R, Inoue M, Minami M, et al. Bilateral mediastinal neurofibroma of the vagus nerves in a patient with neurofibromatosis type 1. Ann Thorac Cardiovasc Surg 2013;19:293-6. [Crossref] [PubMed]
- Shintani Y, Ohta M, Hazama K, et al. Bilateral cervicomediastinal neurofibroma originating from the vagal nerve in a patient with von Recklinghausen's disease: report of a case. Surg Today 2002;32:1068-71. [Crossref] [PubMed]
- Newman A, So SK. Bilateral neurofibroma of the intrathoracic vagus associated with von Recklinghausen's disease. Am J Roentgenol Radium Ther Nucl Med 1971;112:389-92. [Crossref] [PubMed]
- Nachira D, Ismail M, Meacci E, et al. Uniportal vs. triportal video-assisted thoracic surgery in the treatment of primary pneumothorax-a propensity matched bicentric study. J Thorac Dis 2018;10:S3712-9. [Crossref] [PubMed]
- Ismail M, Nachira D. Devising the guidelines: the concept of uniportal video-assisted thoracic surgery-instrumentation and operatory room staff. J Thorac Dis 2019;11:S2079-85. [Crossref] [PubMed]
- Batirel H. Uniportal VATS Approach in Esophageal Cancer - How to Do It Update. Front Surg 2022;9:844796. [Crossref] [PubMed]
- Nachira D, Meacci E, Congedo MT, et al. eComment. Uniportal VATS: the great potential of the technique. Interact Cardiovasc Thorac Surg 2017;25:163. [Crossref] [PubMed]
Cite this article as: Napolitano AG, Nachira D, Vita ML, Leoni C, Margaritora S. Uniportal-video-assisted thoracoscopic surgery (VATS) resection of multiple left thoracic neurofibromas: a case report. Shanghai Chest 2023;7:17.