Pneumothorax as a result of bronchoscopic lung volume reduction with endobronchial valves: a clinical practice review of risk and management strategies
Introduction
As described in the National Emphysema Treatment Trial (NETT), lung volume reduction surgery (LVRS) improved exercise capacity, but a survival benefit was only observed for the subset of patients with both upper-lobe predominant emphysema and low baseline exercise capacity. The subset of patients without upper lobe predominant emphysema and high baseline exercise capacity was found to have an increased mortality along with negligible functional gain (1). Due to the risk of post-operative complications, patients may be reluctant to pursue a surgical approach. As a result, several minimally invasive bronchoscopic methods have been investigated, including bronchial valves, coils, thermal vapor ablation, sealants, and airway bypass stents. Currently, of these options, only endobronchial valves are approved for use in the United States. Commercially available Zephyr® endobronchial valve (Pulmonx Corp., Redwood City, CA, USA) and the intrabronchial Spiration® Valve System (Olympus, Tokyo, Japan) are commonly used for bronchoscopic lung volume reduction (BLVR). The LIBERATE, TRANSFORM, IMPACT, and STELVIO studies evaluated the outcomes of the Zephyr® valve system (2-5), while the EMPROVE and REACH studies evaluated the outcomes of the Spiration® Valve System (6,7). The studies concluded that there was improved lung function, exercise capacity, dyspnea, and quality of life after BLVR with either of the valve systems. Since 2019, BLVR utilizing valves is suggested as a treatment strategy in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Level A evidence) for patients with severe emphysema with hyperinflation and no collateral ventilation between the target and ipsilateral lobe(s) (8,9). More recently, in a study of 1,471 patients, the median survival of patients who underwent treatment with BLVR was found to be longer (1.7 years) compared with those who did not receive the treatment, suggesting that BLVR in patients with severe hyperinflation may also lead to a survival benefit. Notably, BLVR in this study was performed using both valves and coils and was found to be an independent predictor of survival after adjusting for other survival-influencing factors such as age, gender, or severity of disease (10).
However, there are potential complications of the BLVR procedure with valves. While the most common of these is pneumothorax, other complications have also been described including chronic obstructive pulmonary disease (COPD) exacerbation, pneumonia, hemoptysis, valve migration/expectoration, and granulation tissue formation (3-5,11-13). Notably, based on other major trials, these other complications did not show significantly higher prevalence in patients treated with valves compared with controls (3,5,11-13). This review will summarize pertinent studies and expert statements discussing BLVR related pneumothorax risk, evaluation, and management.
Technical aspects of endobronchial valve placement
Patient selection
Candidates for BLVR should undergo a thorough evaluation and meet certain criteria prior to consideration of the procedure. Patients should receive optimal medical therapy as defined by the GOLD guidelines, participate in a pulmonary rehabilitation or comparable physical therapy program, and have quit smoking for at least 4 months. Evaluation should include a full medical assessment, complete lung function measurements, high resolution computed tomography (HRCT) scan, transthoracic echocardiogram (TTE), arterial blood gas (ABG) on room air, and a 6-minute walk test (6MWT). Patients who may be eligible for endobronchial valves must have severe emphysema (GOLD stages 3 and 4 with an FEV1 of 15–45%) with hyperinflation [residual volume (RV) >175% predicted in heterogenous emphysema (EMPROVE study was 150%), RV >200% predicted in homogenous emphysema (only Zephyr approved in the US for homogenous disease)] and absence of collateral ventilation between the target and ipsilateral lobe(s) as can be reviewed in Table 1 (2,6). Additionally, eligible patients should not have any contraindications including inability to tolerate a bronchoscopic procedure, severe gas exchange impairment [PaO2 ≤45 mmHg on room air, PaCO2 ≥50 mmHg on room air, diffusing capacity of carbon monoxide (DLCO) <20% predicted], frequent exacerbations, suspicious pulmonary nodules, large bullae occupying more than one-third of the lung, severe heart failure, uncontrolled pulmonary hypertension, or any allergy to the valve material (14-16). In the case of multiple possible target lobes, nuclear scanning can be performed to identify the lobe with the least perfusion to minimize the amount of ventilation/perfusion (V/Q) mismatching that occurs with lobar occlusion after valve placement (17,18).
Table 1
| Evaluation/testing | Result |
|---|---|
| Clinical history | Symptomatic emphysema despite optimal medical therapy |
| Complete participation in pulmonary rehabilitation program | |
| Clinically stable on ≤20 mg prednisone (or equivalent)/day | |
| Smoking cessation for ≥4 months | |
| Able to tolerate anesthesia/sedation-based procedure | |
| BMI <35 kg/m2 | |
| Pulmonary function tests | FEV1 15–45% predicted |
| RV ≥175% predicted | |
| TLC ≥100% predicted | |
| 6MWD between 100–500 m | |
| DLCO >20% predicted | |
| Imaging | Emphysema on CT scan |
| Collateral ventilation | Target lobe must have absence of collateral ventilation |
BLVR, bronchoscopic lung volume reduction; BMI, body mass index; FEV1, forced expiratory volume in 1 second; RV, residual volume; TLC, total lung capacity; 6MWD, 6-minute walk distance; DLCO, diffusing capacity of carbon monoxide; CT, computed tomography.
Valve sizing and placement
The Zephyr® valve system utilizes a HRCT for initial screening which is uploaded and analyzed. Afterwards, a StratX® report is generated based on the computed tomography (CT) analysis, reporting emphysema severity, fissure integrity, and heterogeneity. The Zephyr® valve is a one-way valve that has a duckbill design (Figure 1A) and is made of a self-expanding nitinol frame with a silicone cover that exerts radial force against the airway wall. There are two valve sizes (4.0 and 5.5 mm diameter) which both expand by 3 mm with the option for a “low-profile” (LP) version that has a reduced length (14). Valve sizing is determined using sizing wings on the deployment catheter which approximates the size of the airway diameter and measurement marks to determine length. Use of the Chartis® (Pulmonx Inc., Redwood City, CA, USA) system which uses a balloon occlusive catheter system to measure distal airflow is recommended intra-procedurally to evaluate for the presence of collateral ventilation prior to valve insertion for patients who have been found to have partially complete (80–95%) fissures on the StratX® report (15,19). The valves are then deployed using a catheter system that extends through a 2.8 mm bronchoscope working channel.
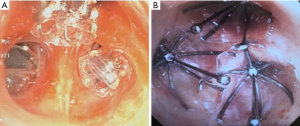
The Spiration® Valve System recommends patients receive a HRCT scan as part of the evaluation for BLVR. This scan gets uploaded for screening by SeleCT®, a quantitative tomography system, which provides a report of emphysema severity, fissure integrity, and heterogeneity. The valve has an umbrella design (Figure 1B), which allows for air and mucus to drain, and is made of nitinol supporting a polyurethane cover with anchor tips at the base to prevent valve migration. The valve is also designed with a removal rod to assist with retrieval and is available in sizes from 5.0–9.0 mm. Valve sizing is determined using a preoperatively calibrated balloon catheter that is then used during the procedure to evaluate airway diameter. Deployment requires unsheathing of the valve via a catheter that extends through a 2.8 mm bronchoscope working channel (6).
Pneumothorax and persistent air leak after BLVR
Pneumothorax occurs in up to 34% of treated patients due to compensatory overexpansion of the ipsilateral untreated lobe(s) (2,17). A meta-analysis by Labarca and colleagues, found an incidence of pneumothorax of 26% (79/293 patients) with no difference in the rate found in subgroup analysis based on emphysema distribution (20). Notably, there has been a higher prevalence of the development in pneumothorax after BLVR in recent studies, likely because of better patient selection when assessing for the absence of collateral ventilation (3,5,13). As a result, there has been debate as to whether pneumothorax post valve placement should even be considered a true complication or a treatment effect. A retrospective analysis by Gompelmann and colleagues of 70 patients with pneumothorax after BLVR found that this complication generally has no negative impact on long term clinical status (21). Another large retrospective analysis by Gompelmann and colleagues found that the occurrence of pneumothorax did not influence survival (22). The LIBERATE study also did not find differences in outcomes for patients who developed a pneumothorax compared with those that did not (2). In another multicenter study by Fiorelli and colleagues of 423 treated patients treated with endobronchial valves for severe heterogeneous emphysema (17.3% pneumothorax rate), the authors concluded that complications do not seem to have a significant impact on clinical outcomes in patients who achieve lobar atelectasis (23). Though pneumothorax is of significant concern after BLVR, Gompelmann and colleagues noted that most cases resolved either with observation or with the insertion of a chest tube. These patients with resolved pneumothorax showed substantial reductions in the target lobar volume at follow up (24). Adequate lung volume reduction with greater than 50% reduction in target lobar volume has demonstrated clinically significant benefits in hyperinflation, exercise capacity, quality of life, and airflow obstruction. An approach with complete lobar occlusion and a higher risk of pneumothorax may be superior to incomplete lobar occlusion when considering patient outcomes (25,26). These studies suggest that pneumothorax should be considered as a possible treatment effect with no bearing on outcomes or expected benefits.
Mechanism of pneumothorax after BLVR
The mechanism of pneumothorax after BLVR is secondary to the ipsilateral non-target lobe rapid expansion which can contribute to an injury in that lobe resulting in an alveolar or bronchopleural fistula (27). An alternate mechanism is a pneumothorax ex-vacuo. In this scenario, a vacuum develops in the pleural space surrounding the collapsed lobe and gas from ambient tissue and blood are subsequently drawn into the pleural space. However, in this case, the seal between the visceral and parietal pleura remains intact and patients are generally asymptomatic with pleural air expected to resolve spontaneously over time without need for chest tube insertion (28,29). A pneumothorax might also theoretically originate from a barotrauma response to the acute volume reduction in the treated lobe. In this instance, the valves may be closing the originating bronchi which may prevent an air leak. These cases of pneumothorax are expected to be less extensive on chest X-ray, and patients may also be relatively asymptomatic (17).
Risk factors for the development of pneumothorax after BLVR
Several studies have looked at identifying risk factors for the development of pneumothorax after BLVR. Such risk factors have been found to include patients with significant pleural adhesions in the target lung, significant paraseptal emphysema, large difference in volume of the target lobe compared with the ipsilateral lobe(s), high emphysematous destruction score of the ipsilateral nontreated lobe(s), and rapid onset of atelectasis (17). The risk is the highest in the first 72 hours post procedure, and hence, it is recommended that patients get admitted to the hospital for observation post procedure for 48–72 hours (23). Pneumothorax as a complication from BLVR has been seen more frequently in patients with valve placement in the left upper lobe. For example, the rates of right and left side upper lobe pneumothorax were reported as 8% and 52%, respectively in the TRANSFORM study, while 6.3% and 66.4%, respectively, in the LIBERATE study (2,3). However, comparatively, in a study by Fiorelli and colleagues in 36 procedures which were followed up for 5 years, despite left upper lobe treatment in 72%, pneumothorax complication was only seen in 6% (30). Another risk factor to consider is whether BLVR was performed on the most diseased lobe. A post-hoc analysis of data from the LIBERATE trial found that patients who were not treated in the most diseased lobe had worse outcomes with a higher risk of a complex pneumothorax (which was defined as leading to death or requiring valve removal) (2).
Rupture of blebs, bullae, and fragile lung tissue in the ipsilateral non-treated lobe are believed to be important contributing causes to the development of pneumothorax (31). While clinical trials did not find an elevated pneumothorax risk in patients with paraseptal bullae or blebs, an expert panel noted that this could be influenced by selection bias as these patients were commonly excluded from participating in trials investigating valve treatment (17). Interestingly, successful cases using endobronchial valves for management of bullae have been described (32-35). Having ipsilateral surgery or interventions with resultant pleural adhesions or having de-novo pleural adhesions in the untreated lobe can lead to a higher rate of pneumothorax. A study by van Geffen and colleagues demonstrated that patients with a higher pleural adhesion score based on a pre-intervention HRCT suffered a higher rate of pneumothorax after BLVR (36). Authors from this study recommend that a prolonged observation time in the hospital or serial chest X-ray may be helpful. The presence of pleural adhesions, however, has not consistently shown to be associated with a higher rate of pneumothorax as noted by Gompelmann and colleagues in the post-hoc analysis of the LIBERATE trial (37). Nevertheless, an expert panel does regard the presence of pleural adhesions an important risk factor after valve treatment (17). A prospective study evaluating pleural adhesion using ultrasound or HRCT may be beneficial.
The risk of pneumothorax is reportedly higher when upper lobes are treated in general when compared to the lower lobes. Two trials demonstrated a nonsignificant trend toward more pneumothoraces when treating upper lobes as opposed to lower lobes (13,26). However, there was no consensus within the expert panel regarding this possible risk factor (17).
Gompelmann and colleagues identified low attenuation volume of the ipsilateral untreated lobe, ipsilateral untreated lobe volume/hemithorax volume ratio, emphysema type, pleural adhesions, and RV as significant predictors of pneumothorax (37). However, no single risk factor has been identified that could influence decision making as to whether a patient should be treated or excluded from valve therapy. A thorough work up during the patient selection process and identifying risk factors such as a high destruction score in the non-treated ipsilateral lobe, target lobe volume to total lung volume ratio, RV, structural deformities as described would be helpful to predict outcomes and guide physicians in discussion with patients regarding proceeding with BLVR.
Reducing risk of pneumothorax after BLVR
In a multicenter, randomized, sham procedure controlled double-blind trial, bilateral-partial lobar bronchial valve occlusion of both upper lobes of patients with severe emphysema was found to have shifted lung volume from the treated lobes to the untreated lobes to a significant degree. Though device safety was confirmed and pneumothorax was found to be reduced, this trial did not achieve clinically meaningful results (38). Similarly, in a randomized, blinded, and sham procedure-controlled study Ninane and colleagues demonstrated that incomplete occlusion was safe without reports of pneumothorax but not effective in the majority of patients (39). In a retrospective study, Egenod and colleagues found that a two-staged approach to BLVR can lead to progressive lung volume reduction as well as reduced pneumothorax risk. Initially, valves were placed in all but the most proximal segment or sub-segment with the remaining segment treated in a follow up procedure with only 4 of 58 (7%) of treated patients suffering a pneumothorax. However, in this study, only 31% of patients had complete atelectasis (40). To date, there is no definitive BLVR procedural modification that is recommended to reduce pneumothorax risk.
Physical strain and cough have been hypothesized as possible causes for spontaneous pneumothorax. One retrospective analysis by Herzog and colleagues reported that cough suppressants and bed rest for 48 hours post BLVR reduced the rate of post procedural pneumothorax from 25% to 5% (41). However, there is limited data to routinely prescribe this modified medical therapy for all patients post BLVR.
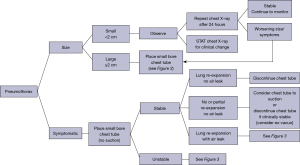
Management of pneumothorax after BLVR
Post procedural care is critical after BLVR and includes hospitalization for at least 3 nights, appropriate staff training for pneumothorax recognition, serial follow up chest X-rays, patient/caregiver education, and an emergency pneumothorax kit available on the ward with all necessary chest tube placement supplies (17). Valipour and colleagues as well as van Dijk and colleagues have developed expert statements and suggest treatment algorithms for patients who develop pneumothorax after BLVR (17,42). A summary of these recommendations can be reviewed in Figures 2,3. In general, first, the size or extent of the pneumothorax is assessed based on the amount of pleural separation. A pneumothorax is determined to be small if there is less than 2 cm of pleural separation or large if there is 2 or more cm of pleural separation. Additionally, a functional assessment is made, whether the patient is symptomatic or asymptomatic. Clinical observation is recommended if a pneumothorax is small and the patient is asymptomatic. Serial chest X-rays to monitor for any progression is recommended in this case. If a pneumothorax is enlarging, or clinical symptoms deteriorate, small-bore chest tube placement is suggested (though, a larger bore chest tube may be required if respiratory failure or subcutaneous emphysema result). A follow up CT scan of the chest may be helpful to localize and evaluate the extent of a pneumothorax as well as guide further treatment options or manage aberrant chest tube placement (17,42).
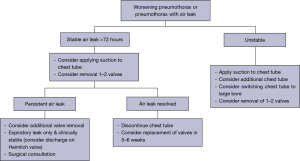
Management of persistent air leak after BLVR
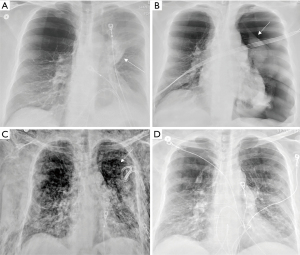
Should a patient develop a post BLVR persistent air leak (considered when an air leak is present for a week or more after chest tube placement), the decision must then be made as to whether one or more bronchial valves should be removed. By removing one or more valves, the target lobe could re-expand with pleural apposition and promote healing of an alveolar or bronchopleural fistula. In the case of valve removal, the most proximal one is suggested to be removed since this will improve airflow throughout the lobe distal to the valve allowing at least partial lobe re-expansion. Additionally, the most proximal valve is likely to be anatomically easiest to replace. If the air leak stops after valve removal and does not reappear after discontinuation of the chest tube, valve replacement can be considered 6–8 weeks after chest tube removal (24,42). If a persistent air leak for at least one week after valve removal occurs, mechanical or chemical pleurodesis, use of a Heimlich valve, or surgical approach should be considered (17,42). Figure 4 demonstrates a case where a left sided pneumothorax developed 7 days after successful BLVR of the left upper lobe. Due to persistent air leak and substantial respiratory symptoms with development of severe subcutaneous emphysema, all the valves were ultimately removed with resolution of the pneumothorax, air leak, and subcutaneous emphysema.
Follow-up after BLVR
Bianchi and colleagues discovered that participation in pulmonary rehabilitation after BLVR yielded further improvement in exercise tolerance (43). To monitor progress after BLVR, quality of life questionnaires, repeat lung function tests with 6-minute walk testing, chest X-ray or CT scan are typically ordered. Additionally, patients are recommended to continue their prior COPD routine with supplemental O2 if needed, bronchodilator/respiratory treatments, exercise, and preventive care (including vaccinations) (44).
Conclusions
BLVR has been found to be a safe and reversible procedure which is effective at improving the lung function, symptoms, quality of life, and possibly even mortality of patients affected by severe emphysema. Additionally, the use of valves for BLVR is not prohibitive for future LVRS or lung transplant. Pneumothorax is the most common and concerning complication after BLVR and there are recommended strategies to evaluate and manage this occurrence. Initially, careful preprocedural evaluation of patients including assessment of risk for the development of complications is an important part of patient selection. Post valve care with hospitalization for at least 3 nights with easy access to chest tube supplies is critical should a pneumothorax develop during this period. Patients and hospital staff should be educated in ensuring prompt identification should such a complication arise. Finally, managing a BLVR related pneumothorax and persistent air leak requires a thoughtful and stepwise approach to both avoid and mitigate this complication to achieve the best result for the patient. Expert statements have been published to help guide clinicians’ assessment and management of this post procedural complication. Further research is needed to help identify and manage risk factors to help reduce the development of pneumothorax after BLVR.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-34/coif). DKH serves as an unpaid editorial board member of Shanghai Chest. DKH is a consultant for Pulmonx, Olympus, Eolo, and Deerfield Catalyst. He is also a speaker for Pulmonx. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Criner GJ, Sue R, Wright S, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198:1151-64. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Klooster K, Hartman JE, Ten Hacken NH, et al. One-Year Follow-Up after Endobronchial Valve Treatment in Patients with Emphysema without Collateral Ventilation Treated in the STELVIO Trial. Respiration 2017;93:112-21. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Criner GJ, Delage A, Voelker K, et al. Improving Lung Function in Severe Heterogenous Emphysema with the Spiration Valve System (EMPROVE). A Multicenter, Open-Label Randomized Controlled Clinical Trial. Am J Respir Crit Care Med 2019;200:1354-62. [Crossref] [PubMed]
- Li S, Wang G, Wang C, et al. The REACH Trial: A Randomized Controlled Trial Assessing the Safety and Effectiveness of the Spiration® Valve System in the Treatment of Severe Emphysema. Respiration 2019;97:416-27. [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. [Crossref] [PubMed]
- Hartman JE, Welling JBA, Klooster K, et al. Survival in COPD patients treated with bronchoscopic lung volume reduction. Respir Med 2022;196:106825. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017;93:138-50. [Crossref] [PubMed]
- Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2019. Respiration 2019;97:548-57. [Crossref] [PubMed]
- Hartman JE, Vanfleteren LEGW, van Rikxoort EM, et al. Endobronchial valves for severe emphysema. Eur Respir Rev 2019;28:180121. [Crossref] [PubMed]
- van Dijk M, Sue R, Criner GJ, et al. Expert Statement: Pneumothorax Associated with One-Way Valve Therapy for Emphysema: 2020 Update. Respiration 2021;100:969-78. [Crossref] [PubMed]
- Kristiansen JF, Perch M, Iversen M, et al. Lobar Quantification by Ventilation/Perfusion SPECT/CT in Patients with Severe Emphysema Undergoing Lung Volume Reduction with Endobronchial Valves. Respiration 2019;98:230-8. [Crossref] [PubMed]
- Koster TD, van Rikxoort EM, Huebner RH, et al. Predicting Lung Volume Reduction after Endobronchial Valve Therapy Is Maximized Using a Combination of Diagnostic Tools. Respiration 2016;92:150-7. [Crossref] [PubMed]
- Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic Lung Volume Reduction with Endobronchial Zephyr Valves for Severe Emphysema: A Systematic Review and Meta-Analysis. Respiration 2019;98:268-78. [Crossref] [PubMed]
- Gompelmann D, Benjamin N, Kontogianni K, et al. Clinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valves. Int J Chron Obstruct Pulmon Dis 2016;11:3093-9. [Crossref] [PubMed]
- Gompelmann D, Benjamin N, Bischoff E, et al. Survival after Endoscopic Valve Therapy in Patients with Severe Emphysema. Respiration 2019;97:145-52. [Crossref] [PubMed]
- Fiorelli A, D'Andrilli A, Bezzi M, et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study. J Thorac Dis 2018;10:S3315-25. [Crossref] [PubMed]
- Gompelmann D, Herth FJ, Slebos DJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration 2014;87:485-91. [Crossref] [PubMed]
- Valipour A, Herth FJ, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014;43:387-96. [Crossref] [PubMed]
- Eberhardt R, Gompelmann D, Schuhmann M, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012;142:900-8. [Crossref] [PubMed]
- Brown MS, Kim HJ, Abtin FG, et al. Emphysema lung lobe volume reduction: effects on the ipsilateral and contralateral lobes. Eur Radiol 2012;22:1547-55. [Crossref] [PubMed]
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996;110:1102-5. [Crossref] [PubMed]
- de Oliveira HG, Macedo-Neto AV, John AB, et al. Transbronchoscopic pulmonary emphysema treatment: 1-month to 24-month endoscopic follow-up. Chest 2006;130:190-9. [Crossref] [PubMed]
- Fiorelli A, Santoriello C, De Felice A, et al. Bronchoscopic lung volume reduction with endobronchial valves for heterogeneous emphysema: long-term results. J Vis Surg 2017;3:170. [Crossref] [PubMed]
- Shen KR, Cerfolio RJ. Decision making in the management of secondary spontaneous pneumothorax in patients with severe emphysema. Thorac Surg Clin 2009;19:233-8. [Crossref] [PubMed]
- Hou G, Wang W, Wang QY, et al. Bronchoscopic bullectomy with a one-way endobronchial valve to treat a giant bulla in an emphysematic lung: a case report. Clin Respir J 2016;10:657-60. [Crossref] [PubMed]
- Lee EG, Rhee CK. Bronchoscopic lung volume reduction using an endobronchial valve to treat a huge emphysematous bullae: a case report. BMC Pulm Med 2019;19:92. [Crossref] [PubMed]
- Santini M, Fiorelli A, Vicidomini G, et al. Endobronchial treatment of giant emphysematous bullae with one-way valves: a new approach for surgically unfit patients. Eur J Cardiothorac Surg 2011;40:1425-31. [Crossref] [PubMed]
- Tian Q, An Y, Xiao BB, et al. Treatment of giant emphysamous bulla with endobronchial valves in patients with chronic obstructive pulmonary disease: a case series. J Thorac Dis 2014;6:1674-80. [PubMed]
- van Geffen WH, Klooster K, Hartman JE, et al. Pleural Adhesion Assessment as a Predictor for Pneumothorax after Endobronchial Valve Treatment. Respiration 2017;94:224-31. [Crossref] [PubMed]
- Gompelmann D, Lim HJ, Eberhardt R, et al. Predictors of pneumothorax following endoscopic valve therapy in patients with severe emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:1767-73. [Crossref] [PubMed]
- Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol 2014;21:288-97. [Crossref] [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [Crossref] [PubMed]
- Egenod T, Tricard J, Fumat R, et al. Two-Stage Bronchoscopic Endobronchial Valve Treatment Can Lead to Progressive Lung Volume Reduction and May Decrease Pneumothorax Risk. Int J Chron Obstruct Pulmon Dis 2021;16:1957-65. [Crossref] [PubMed]
- Herzog D, Poellinger A, Doellinger F, et al. Modifying Post-Operative Medical Care after EBV Implant May Reduce Pneumothorax Incidence. PLoS One 2015;10:e0128097. [Crossref] [PubMed]
- Valipour A, Slebos DJ, de Oliveira HG, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema--potential mechanisms, treatment algorithm, and case examples. Respiration 2014;87:513-21. [Crossref] [PubMed]
- Bianchi L, Bezzi M, Berlendis M, et al. Additive effect on pulmonary function and disability of intensive pulmonary rehabilitation following bronchoscopy lung volume reduction (BLVR) for severe emphysema. Respir Med 2018;143:116-22. [Crossref] [PubMed]
- Flandes J, Soto FJ, Cordovilla R, et al. Bronchoscopic Lung Volume Reduction. Clin Chest Med 2018;39:169-80. [Crossref] [PubMed]
Cite this article as: Wagh A, Ravikumar N, Hogarth DK. Pneumothorax as a result of bronchoscopic lung volume reduction with endobronchial valves: a clinical practice review of risk and management strategies. Shanghai Chest 2023;7:19.