Selection of the population in lung cancer screening studies: a narrative review
Introduction
Background
Screening in lung cancer should be understood as a measure in the framework of early cancer detection and treatment, as well as a first step of prevention strategies in selected populations (1-4). Selection criteria were mostly the smoking behavior and the age of probands in lung cancer screening studies and corresponding programs. In the last years, the method of choice was low-dose computerized tomography (LDCT) of the chest in most of the screening programs and studies offered (5-7). This method shows good results, but is also connected with the problem of overdiagnosis and subsequently with unnecessary overtreatment. Not only that, it is at the same time an invasive measure which can do some harm in probands and generally causes costs in the national health systems.
Objectives
The aim of new screening studies must be to restrict the population to be screened as a measure to increase effectiveness in lung cancer screening programs, to decrease health and collective detriment and avoidable costs, and in general, raise the clinical practicability of such screening programs (8). Aim must also be, to define the screening population as narrow as possible, with at the same time being as socially accepted and with a high health beneficial expectation. In doing so, the question has to be answered if there are other determinants, as smoking behavior and age, to derive this aim and find ways of modelling and designing such studies and programs (9).
I present the following article in accordance with the Narrative Review reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-21-33/rc).
Methods for the narrative review
Literature of the last ten years was searched to get knowledge about the main influence factors for developing lung cancer. It was looked at the determinant smoking behavior, the influence of age, the occupational and behavioral situation for those who underwent lung carcinogenesis, the radon exposure, the genetic settings and family influence, and the influence of general cancer risks for lung cancer amongst others. For this narrative review the data base MEDLINE of the US National Library of Medicine from the National Institutes of Medicine was used for articles written in English and published in the time period from April 2011 to April 2021 (Tables 1 and 2). The understanding in writing this narrative review was, to give some perspectives how to define a population to be screened in a lung cancer screening study beyond the determinants smoking status and age. And thus, the resulting tables do not want to purport that this is a systematic literature analysis.
Table 1
| Items | Specification |
|---|---|
| Date of search | 10/04/2021 |
| Databases and other sources searched | MEDLINE; US National Library of Medicine |
| Search terms used | Lung screening populations, developing of lung cancer, lung cancer screening models |
| Timeframe | April 2011 to April 2021 |
| Inclusion and exclusion criteria | Peer reviewed, English language |
| Selection process | By the author himself |
Table 2
| Used search terms in the narrative review* |
| Lung cancer screening |
| Development of lung cancer |
| Selection of screening population |
| Lung cancer screening models |
| Smoking behavior |
| Smoker/non-smoker |
| Risks |
| Risk distribution |
| Risk structure |
| Risk models |
| Interfering risks |
| Age |
| Genetic |
| Occupational exposure |
| Radon exposure |
| Behavioral influence |
| General influences |
| General risk factors |
| Score of risks |
| Multiple risks |
| Effectiveness of screening |
| Screening interval |
| Screening costs |
| Screening practicability |
| Screening false negative |
| Screening false positive |
*, the additive term ‘lung cancer’ was used in every search if not already incorporated.
In national lung screening trials as the NLST of the United States it was shown basically that screening results are consistent with the existing literature on screening by means of LDCT and chest radiography and has the power to reduce mortality in lung cancer (10,11). Moreover, it was shown in an accurate model that incorporates additional risk factors in the screening action may identify more persons who have lung cancer or in whom lung cancer will develop if applied to the NLST data (12).
The main determinants are described in detail including their impact on a possible study design. In the above mentioned NLST study solutions are worked out, for the resolving study designs on this basis beginning with lung cancer screening. Lung cancer screening is seen here as a tool showing the connection to general risks to an event. Especially for lung cancer prediction models there is the need to provide sufficiently strong discrimination between individuals with high and low likelihood of being diagnosed with lung cancer in the following years (13,14). For lung cancer screening the selection process of individuals to be screened using prediction models has been described and considerations for future studies have been made (15).
The development of scores of risks is shown as a method to bundle risks in a structured way. Risk structure and its distribution in individuals is another method to model the influence in a study for lung cancer screening, as well as the more general competing risks and the problem of interfering risks. The same applies to the effecting risk for an individual which can be modelled in the design for such a study. All these individual and some more general design features are shown as possible elements in the study design. The resulting screening study population is shown as a function of these study design elements.
Results
Main determinant is smoking behavior
Since more than forty years there is evidence that smoking is one of the most prominent risk factors in the development of lung cancer (16). The percentage of lung cancer patients having a heavy smoking history is in the most countries, including all big nations, quite distinct, and the number of smokers in male Chinese lung cancer population is considerably high (17). So, the smoking behavior often defined by pack years with a given threshold is widely used. In many programs the threshold for an individual is a 20 pack-year smoking history and currently smoking or having quit within the past 15 years (18). Many studies have shown that this threshold is a good compromise to sort out roughly the higher lung cancer risks in the population. In some studies, the more general term tobacco consumption is used, translating other forms of tobacco use into pack years, more associated with cigarette smoking. In China the prevalence of cigarette smoking in male population has a percentage of 52.1% and can be seen as in other industrial developed countries as a backbone risk in developing lung cancer (17,19). Also, the Chinese population of never smokers has to be investigated: Risk factors vary between males and females, the number of determinant risk factors, and the magnitudes of their effects can be different (20).
Despite some changes in manufacturing cigarettes and the use of additive substances for taste and addiction cigarette smoking, general tobacco consumption is a highly hooking habitual practice, not easily changed. Thus, long term tobacco use is quite common and moreover, the individual changes in the respiratory system are long lasting (21). In a meta-analysis of 2018 with 7 million individuals, smoking as a risk factor for lung cancer in women and men was calculated with the pooled multiple-adjusted lung cancer relative risk as 6.99 in women and 7.33 in men, respectively, and a statistically non-significant pooled ratio (22). Obeying these facts, it is absolutely necessary, to look at the smoking history of probands who should be included in a lung cancer screening program. Since for the manifestation of harmful changes in respiratory tract, some time for becoming effective has to be calculated, it is meaningful to set a certain time interval of tobacco-use and its amount. In this light, some 20 pack-year smoking limit is reasonable, which can be adapted slightly in a screening design. It is worth mentioning, that the smoking career often starts at the age of teenagers, such that with the age of 40 to 50 years a long history will already be achieved.
Non-smoking-related lung cancer
In Asia the assessment of selection for LDCT is different from Europe and North-America. Non-smokers related lung cancer are associated strongly with female gender and family history of lung cancer and so it was concluded in a Taiwan population (23,24), that the attention to these factors can improve the efficiency of lung cancer screening programs. Furthermore, it was shown that the modification of lung imaging reporting and data systems (Lung-RADS) improves the performance of LDCT screening in a population with high prevalence of non-smoking-related lung cancer (25,26). In addition, it is recognized that heritable risks and genetic contributions are highly associated with the risk of lung cancer among patients with non–smoking-related lung cancers (11). Therefore, special attention has to be turned on this subpopulation with special attributes and should find input into potential lung cancer risk models.
The determinant age
Among both women and men, the incidence of lung cancer is low in people aged <40 years and increases up to age 75–80 years in most populations (27). In general, the observation that the age-specific incidence curve of many carcinomas is approximately linear on a double logarithmic plot, led amongst others to the description by a multistage model that predicts two basic phases in the age-specific incidence curves, a first exponential phase until the age of about 60 years of age followed by a linear phase after that age (28). Age is an important determinant in selecting the screening population in these programs, since the respiratory tract is part of “filtering system” of all air coming into the human body with some memory of defending harmful or even toxic substances. Repair will play a role, but over a long time the history of the complete lifestyle will be mapped on the tissue of the respiratory system (27). Thus, in higher ages the frequency of lung carcinogenesis is more often seen and more severe cases as well (21). Epidemiological data say, that age alone is only a “mild” factor, milder than the smoking behavior. In combination with smoking an age threshold of 40 to 50 years seems to be reasonable to be taken, since smoking starts in individuals often in early years of 20 or even younger such that 20 and more years of smoking history can be easily reached. Setting the threshold in age too high, such as 55 to 60 years of age, means, that many positive cases could be missed and therefore, torpedo the result. Depending on the life expectation of the whole population the upper bound for screening should not be greater than 80 years of age and should range around the overall median life expectation in the total population, which is also in accordance with general findings (27).
The classical selection of a screening population in lung cancer should thus always take the two determinants smoking behavior and age in a combination into account. Doing this, the most unknown cases will be detected.
Occupational exposure
There are occupational risks which can also do a lot of harm as respirable dust in coal mines and other mining industries, the fine particulate matter and micro dust in farming and production, but also nearby the exhaustion material of machines and vehicles and in the general production, as well as the special long known factor of the asbestos fibers. But also cooks in kitchen facilities, and individuals working in paper manufacturing, printing facilities, asphalt workers, and many more are sometimes exposed to carcinogenic substances being effective in the respiratory tract. These effects have to be seen independently from the smoking determinant but have also a time component which is important to obey. The history is not easily measured quantitively but alone the fact that an individual is working for some years in these industries might be reason enough to be included into the selection process. Since it should be seen as an additive risk it must be treated in the selection process as an “and/or” procedure with some threshold for the years of exposure which should be similar to that of the smoking history. In China programs have been performed using this selection tool successfully (29).
Radon exposure
In some regions the exposure of the gas radon is quite high and it is known to be harmful especially in the respiratory system. Since it is a heavy gas, it is assembling in narrow parts of a house as the basement. This risk factor should be taken as an additional and independent risk factor with a less distinctive weight. The regional character of the appearance of this gas should also be deliberated and can be omitted in regions without this exposure. It can be part of a risk score. Other environmental risks should be summarized under general cancer risks (30).
Behavioral setting
The behavioral influences beside smoking are not so easily categorized because of being so divers and different in intensity (31,32). In China, it is known that the cooking in the households play some additional role in producing aerosols being carcinogenic effective. In general, the influence of the behavioral setting on developing lung cancer is difficult to be anticipated and the risk is not so easy to predict. Alcohol use and the use of other drugs can also be a factor which might not be independent from others as smoking. In a Chinese program the indoor cooking can be taken as an independent additional small selection factor. It could be a factor in yet to be developed score of risks (33).
Genetic setting
Since approximately 10–15% of lung cancers in Western countries occur in never-smokers, and not all can be accounted for the action of occupational and environmental risk factors, genetic factors should be also considered (34,35). The genetic influence in lung cancer is not as distinct as in colon or breast cancer. There are some genetic settings which may be giving a hint of being more expressed in the process of lung cancer. A clinically relevant increased risk of developing lung cancer can have its cause by variants in the TP53 tumor-suppressor gene or e.g., EGFR variants, T790M in particular, cause the EGFR susceptibility syndrome (34). In general, these factors are difficult to be derived and interpreted and therefore it cannot easily be taken as a selection feature in the screening process of lung cancer. The problem is here to figure out risk numbers for a heterogenous population which will have a positive selection impact (36-38).
General cancer risk factors
These risk factors are related to those mentioned above (27). They may be hidden in occupational, life style, and the genetics. The immune status also plays a role as well as the psycho-somatic setting of an individual. Obesity may also be a factor and more generally, diet and the use of alcohol may be taken as examples (39,40). All these factors are difficult to be translated into a numerical risk for selecting a population for lung cancer screening which most benefit of this action. Thus, currently it seems not to be promising to incorporate general risk factors into the selection process as they may not have the weight for a broader risk use.
Screening and the connection to general risks to an event (here lung cancer)
The lung cancer screening process wants to detect individuals having a certain risk to develop a severe lung cancer disease showing already some signs of this disease as precancerous stages and/or mini foci which stands here for the event to be detected (18,41,42). Therefore, attributable risks are used to decrease the number of probands being tested, since these methods are interventional and can cause some harm, for example by using LDCT. Thus, the selection process of these attributable risks is crucial: They must not only have any impact, but the impact must be clearly measurable and easily to be derived. Above possible attributable risks have been named and the problem is, to find some input as a risk figure into a screening model. It is seen that some of the attributable risk factors as smoking history and age of an individual can be taken as a determinant in the screening model whereas other input factors are less strong. A modelling with many risk factors can cause difficulties in performing and will not per se end up with a better detection result and lower costs and harm. A too restricted model will detect little incriminated (pre) cancerous stages and overlook possible positive screening test results. In screening studies with some thousands of probands the strategy has to be tested and evaluated.
Discussion
Score of risks
Risk prediction models incorporating multiple risk factors have been recognized as a method of identifying individuals at high risk of developing lung cancer (43). One possibility for a new defined screening population is to introduce a score of risk factors in adding some independent risks. As far as we know, the risk score method has not been applied in a bigger LDCT lung cancer screening trial. In doing so, a definitive score threshold must be defined before coming into action with the screening intervention. As an example of applying this method in lung cancer diagnosis and treatment, an easy-to-apply risk score has been proposed already, categorizing patients into different risk groups. This was used as a diagnostic measure before treatment start with a PD-1/PD-L1 antibody (44). Thus, for example, a risk score can be taken consisting of the input risks of smoking history, age, occupational exposure, radon exposure, alcohol/drug use and obesity with a defined threshold at which the interventional action takes place. In case of a study, the sample size calculation has to be considered in advance, obeying that there are several input factors with varying impact. The resulting sample size of a study will be greater with more stratified input factors compared to the classical model with smoking status and age. Aim of this selection is to sort out more exactly the higher risks and screen a smaller number of individuals.
Risk structure and its distribution in individuals
In modelling the screening population in a more elaborated way, the underlying risk structure of the individuals is used (45). In this case more than the tobacco consumption and age is in question. From worldwide epidemiological data the risk factors for lung cancer are published, for patients with lung cancer for instance as prognostic factors, e.g. derived by statistical logistic regression models. These data vary from region to region and can be taken with its distribution as an input for the risk structure of attributable risks in a screening model. Thus, the established selection rules in a coalmining area or in a rural region might be different compared to a big mega city, for example. Regional differentiation seems to be pivotal in the screening programs especially in big countries such as China. In Europe with a lot of smaller countries this does not play the same role, there, a nationwide selection rule for the screening population may be sufficient. Important in any case is to know the attributable risks interrelated with the process of lung carcinogenesis and the distribution in the underlying population.
Competing risks and problem of interfering risks
As in other fields of real-life situations the concept of competing risks must be taken into consideration in using a risk profile for population selection. In the case of detecting lung cancer in screening programs the determinants are smoking behavior and age. Risks often interfere with each other as for example the tobacco consumption and the exposure to micro dust or the influence of coal dust in the mining industry. It is difficult to build up interaction models of risks (46-48). One possibility could be to use the mathematical method of manifolds and hyperplanes to depict the interference of risks. In so far, the definition of the screening population can be understood as a solution of an optimization problem. In a first approximation in modelling the risks can be taken as independent. So far, no complex models have been used in bigger lung screening models.
Effecting risk for an individual
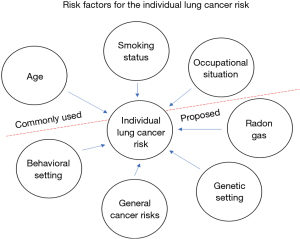
The effect of attributable risks in concern of lung cancer on an individual are highly different (49) and do not happen in the way of causal determinism (Figure 1). The tale of the 90 years old smoker with good health is legend. There is a very high probability that it will not be so, but there is no causal deduction chain that for instance smoking finally will in every case lead to lung cancer. Hence the probabilistic statistical approach in attributable risk assumption to define a screening population is today the way to perform. Therefore, to find the distribution of lung cancer risks in defined regions is a basic research front work.
Screening population
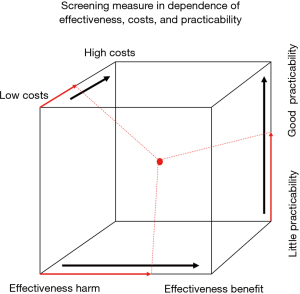
The setting up of a screening population has to be done before the program starts and should not be altered for a longer time period. Thus, making it possible to compare results of findings with the complementary population set which does not undergo some screening interventions (50). With this procedure it is possible to validate the concept taken and to prove the quality of screening actions (Figure 2). The screening measure can be seen in dependence of effectiveness, costs, and practicability. These three parameters can determine the success of the screening action. Clearly the costs are dependent on the number of single measures taken, which is also true for the practicability: Low costs are associated with a small number of those measures, but effectiveness often comes along with a higher number of measures taken. Thus, a balance (an optimum) has to be found in the set of measures (a technical method would be linear optimization). With quality assurance methods the adjustment of screening population selection is then possible. Therefore, it is crucial to work with a well-defined unchanged population in a time limited setting, where the determinant risks are always an indispensable part of it, which is here tobacco use and age of the proband.
Effectiveness of screening and the false negative rate
As insinuated above some quality control and hence also effectiveness must be measured in the screening population. That can be done best in comparing the effect of screening, which are the detected carcinogenic processes as pre-cancerous stages, mini foci, foci, and others and compare it with the findings in the complementary population. In a first approach these findings can be compared to the specific finding in the total population which will be here also the early stage diseases which are by the way often detected incidentally. An issue is also all the undetected (pre-) cancerous states in the screening course which has its expression in the false negative rate, but should be a minor problem (51). Effectiveness should be measured in the frame of statistical analysis plans given in advance and determine the time points, or points of events when the measurement of the effect should take place.
Radiation harm and the problem of the false positives
In measuring the effect of screening, the benefit will be the detected (pre-) lung cancers. There may be also some sources of irritation in the medical frame which are translated into confounding statistical factors (52). Especially in using a computerized tomography, the X-ray radiation exposure must be considered. In most models this perturbation is treated more globally and summed up in the radiation burden during the course of screening. Another problem is the psychological situation of those probands which are false positive, believing that they got cancer. This is difficult to measure and not easy to quantify (53). Overdiagnosis and overtreatment is strongly connected to the false positive rate, and in treating slowly growing tumors, which would especially in the elderly subpopulation never become effective as a lethal disease.
Costs and practicability
The costs of screening are directly dependent on the definition of the tested population and hence, the number of probands. Therefore, the number of individuals screened should be as narrow as possible with at the same time detecting as many patients as possible bearing already some early stages of lung tumors (54). The actions taken during the screening process are of course also crucial for the resulting costs, as the repetition number of the screening act. Costs assessment should be an integrative part of the effectiveness evaluation and the costs should finally relate to the benefit of the whole program.
The time interval of screening actions also matters (55) and has to be seen in the light of the measures taken. Appropriate screening intervals in LDCT lung cancer screening as of the NLST have been applied as annual LDCTs, plus shorter-term follow-up LDCTs when indicated (56). It has been suggested that longer intervals could be used for individuals who are at lower risk of lung cancer, but it is difficult to assign these individuals (57).
The Screening process should be as simple as possible and at the same time clinical practicability should be given (58). The medical sources must be proven and in a wide range available. There are other potential screening modalities as sputum cytology, simple chest radiography, or measurement of biomarker levels. None of these has been in total as effective as LDCT. The modality LDCT truly is a cost factor, but if the screened population is chosen in a proper way, the beneficial effect will be worth it.
Summary
In defining the screening population, the two determinants of smoking behavior and age of the probands should be implicitly used. Often it will be indicated, to obey also the occupational situation in the risk model used for screening. Since the regional differences are great, the risk profile and distribution for developing lung cancer should be studied in detail and some of the found independent risks can be added, as, e.g., the exposure to radon gas and micro dust. For lung cancer screening the selection process of individuals to be screened using prediction models has been successfully described and considerations for future studies have been made. So far till now, more complicated risk models have not been broadly used worldwide in lung cancer screening, and so the proof of evidence as for risk score models are pending. A method of proof before using it generally in primary care, would be the conducting of elaborated computerized simulation studies to test the performance of these screening settings.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wieland Voigt and Helmut Prosch) for the series “Lung Cancer Screening” published in Shanghai Chest. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-21-33/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-21-33/coif). The series “Lung Cancer Screening” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheng YI, Davies MPA, Liu D, et al. Implementation planning for lung cancer screening in China. Precision Clin Med 2019;2:13-44. [Crossref]
- Yang D, Liu Y, Bai C, et al. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett 2020;468:82-7. [Crossref] [PubMed]
- Key statistics for lung cancer. American Cancer Society. Accessed January 15, 2021. Available online: http://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Wu FZ, Huang YL, Wu YJ, et al. Prognostic effect of implementation of the mass low-dose computed tomography lung cancer screening program: a hospital-based cohort study. Eur J Cancer Prev 2020;29:445-51. [Crossref] [PubMed]
- Teles GBDS, Macedo ACS, Chate RC, et al. LDCT lung cancer screening in populations at different risk for lung cancer. BMJ Open Respir Res 2020;7:e000455. [Crossref] [PubMed]
- Ji G, Bao T, Li Z, et al. Current lung cancer screening guidelines may miss high-risk population: a real-world study. BMC Cancer 2021;21:50. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Wu FZ, Kuo PL, Wu CC, et al. The impact of patients’ preferences on the decision of low-dose computed tomography lung cancer screening. Transl Lung Cancer Res 2018;7:S236-8. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Tammemägi MC. Application of risk prediction models to lung cancer screening. J Thorac Imaging 2015;30:88-100. [Crossref] [PubMed]
- Kaaks R, Hüsing A, Fortner RT. Selecting high-risk individuals for lung cancer screening; the use of risk prediction models vs. simplified eligibility criteria. Ann Transl Med 2017;5:406. [Crossref] [PubMed]
- Tammemägi MC. Selecting lung cancer screenees using risk prediction models-where do we go from here. Transl Lung Cancer Res 2018;7:243-53. [Crossref] [PubMed]
- Loeb LA, Ernster VL, Warner KE, et al. Smoking and lung cancer: an overview. Cancer Res 1984;44:5940-58. [PubMed]
- Parascandola M, Xiao L. Tobacco and the lung cancer epidemic in China. Transl Lung Cancer Res 2019;8:S21-30. [Crossref] [PubMed]
- Jonas D, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: An evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 198. Agency for Healthcare Research and Quality; 2021. AHRQ publication 20-05266-EF-1.
- Liu X, Yu Y, Wang M, et al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990-2017. Cancer Commun (Lond) 2020;40:611-9. [Crossref] [PubMed]
- Liang D, Wang J, Li D, et al. Lung ccancer in never-smokers: A multicenter case-control study in North China. Front Oncol 2019;9:1354. [Crossref] [PubMed]
- Stevens C, Smith SG, Quaife SL, et al. Interest in lifestyle advice at lung cancer screening: Determinants and preferences. Lung Cancer 2019;128:1-5. [Crossref] [PubMed]
- O'Keeffe LM, Taylor G, Huxley RR, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open 2018;8:e021611. [Crossref] [PubMed]
- Wu FZ, Huang YL, Wu CC, et al. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin Lung Cancer 2016;17:e45-56. [Crossref] [PubMed]
- Lin KF, Wu HF, Huang WC, et al. Propensity score analysis of lung cancer risk in a population with high prevalence of non-smoking related lung cancer. BMC Pulm Med 2017;17:120. [Crossref] [PubMed]
- Hsu HT, Tang EK, Wu MT, et al. Modified Lung-RADS Improves Performance of Screening LDCT in a Population with High Prevalence of Non-smoking-related Lung Cancer. Acad Radiol 2018;25:1240-51. [Crossref] [PubMed]
- Naidich DP. Low Dose Lung CT Screening in an Asian Population. Acad Radiol 2018;25:1237-9. [Crossref] [PubMed]
- Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889-902. [Crossref] [PubMed]
- Meza R, Jeon J, Moolgavkar SH, et al. Age-specific incidence of cancer: Phases, transitions, and biological implications. Proc Natl Acad Sci U S A 2008;105:16284-9. [Crossref] [PubMed]
- Wei MN, Su Z, Wang JN, et al. Performance of lung cancer screening with low-dose CT in Gejiu, Yunnan: A population-based, screening cohort study. Thorac Cancer 2020;11:1224-32. [Crossref] [PubMed]
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: col-laborative analysis of individual data from 13 European case-control studies. BMJ 2005;330:223. [Crossref] [PubMed]
- Li YH, Shieh SH, Chen CY. The influence of health behaviors on survival in lung cancer patients in Taiwan. Jpn J Clin Oncol 2011;41:365-72. [Crossref] [PubMed]
- Klein WM, Bloch M, Hesse BW, et al. Behavioral research in cancer prevention and control: a look to the future. Am J Prev Med 2014;46:303-11. [Crossref] [PubMed]
- Chen TY, Fang YH, Chen HL, et al. Impact of cooking oil fume exposure and fume extractor use on lung cancer risk in non-smoking Han Chinese women. Sci Rep 2020;10:6774. [Crossref] [PubMed]
- Benusiglio PR, Fallet V, Sanchis-Borja M, et al. Lung cancer is also a hereditary disease. Eur Respir Rev 2021;30:210045. [Crossref] [PubMed]
- Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never-smokers – a review. Eur J Cancer 2012;48:1299-311. [Crossref] [PubMed]
- de Alencar VTL, Formiga MN, de Lima VCC. Inherited lung cancer: a review. Ecancermedicalscience 2020;14:1008. [PubMed]
- Kanwal M, Ding XJ, Cao Y. Familial risk for lung cancer. Oncol Lett 2017;13:535-42. [Crossref] [PubMed]
- Wang J, Liu Q, Yuan S, et al. Genetic predisposition to lung cancer: comprehensive literature integration, meta-analysis, and multiple evidence assessment of candidate-gene association studies. Sci Rep 2017;7:8371. [Crossref] [PubMed]
- Lam TK, Gallicchio L, Lindsley K, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:184-95. [Crossref] [PubMed]
- Bandera EV, Freudenheim JL, Vena JE. Alcohol consumption and lung cancer a review of the epidemiologic evidence. Cancer Epidemiol Biomarkers Prev 2001;10:813-21. [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for Lung Cancer. Chest 2013;143:e78S-e92S. [Crossref] [PubMed]
- Moyer VAU.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [Crossref] [PubMed]
- Marcus MW, Raji OY, Field JK. Lung cancer screening: identifying the high risk cohort. J Thorac Dis 2015;7:S156-62. [PubMed]
- Diem S, Fässler M, Hasan Ali O, et al. Risk score for non-small cell lung cancer patients starting checkpoint inhibitor treatment. Cancer Manag Res 2018;10:5537-44. [Crossref] [PubMed]
- Alberg AJ, Brock AV, Ford JG, et al. Epidemiology of Lung Cancer. Chest 2013;143:e1S-e29S. [Crossref] [PubMed]
- Tan KS, Eguchi T, Adusumilli PS. Competing risks and cancer-specific mor-tality: why it matters. Oncotarget 2018;9:7272-3. [Crossref] [PubMed]
- Chappell R. Competing risk analyses: how are they different and why should you care? Clin Cancer Res 2012;18:2127-9. [Crossref] [PubMed]
- Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical com-munity: irrelevance or ignorance? Stat Med 2012;31:1089-97. [Crossref] [PubMed]
- Alberg AJ, Nonemaker J. Who is at high risk for lung cancer? Population-level and individual-level perspectives. Semin Respir Crit Care Med 2008;29:223-32. [Crossref] [PubMed]
- Marcus MW, Raji OY, Field JK. Lung cancer screening: identifying the high risk cohort. J Thorac Dis 2015;7:S156-62. [PubMed]
- Bartlett EC, Silva M, Callister ME, et al. False-Negative Results in Lung Cancer Screening—Evidence and Controversies. J Thorac Oncol 2021;16:912-21. [Crossref] [PubMed]
- Pinsky PF. Assessing the benefits and harms of low-dose computed tomography screening for lung cancer. Lung Cancer Manag 2014;3:491-8. [Crossref] [PubMed]
- Lazris A, Roth AR. Lung Cancer Screening: Pros and Cons. Am Fam Physician 2019;99:740-2. [PubMed]
- Edelman Saul E, Guerra RB, Edelman Saul M, et al. The challenges of imple-menting low-dose computed tomography for lung cancer screening in low- and mid-dle-income countries. Nat Cancer 2020;1:1140-52. [Crossref] [PubMed]
- Pastorino U, Sverzellati N, Sestini S, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer 2019;118:142-8. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M. Appropriate screening intervals in low-dose CT lung cancer screening. Transl Lung Cancer Res 2018;7:281-7. [Crossref] [PubMed]
- Zhang L, Yip R, Jirapatnakul A, et al. Lung cancer screening intervals based on cancer risk. Lung Cancer 2020;149:113-9. [Crossref] [PubMed]
- Ten Haaf K, Tammemägi MC, Bondy SJ, et al. Performance and Cost-Effectiveness of Computed Tomography Lung Cancer Screening Scenarios in a Population-Based Setting: A Microsimulation Modeling Analysis in Ontario, Canada. PLoS Med 2017;14:e1002225. [Crossref] [PubMed]
Cite this article as: Pilz LR. Selection of the population in lung cancer screening studies: a narrative review. Shanghai Chest 2022;6:12.