Iatrogenic oesophageal perforation
Introduction
Oesophageal perforation is an uncommon emergency but is associated with high morbidity and mortality (1-3). Known causes include iatrogenic, spontaneous, traumatic and foreign bodies. Iatrogenic causes are the most common (3), partly due to the increasing use of endoscopic screening programmes and advancements in therapeutic endoscopy.
Although there is no consensus on optimal management strategy, it is well established that prompt diagnosis and treatment within 24 hours of the perforation is associated with improved outcomes (1,3-8). In a metanalysis of 75 studies, Biancari et al. (8) found a pooled mortality of 11.9%, however patients treated within 24 hours had a mortality of 7.4%, whilst those treated after 24 hours had a mortality of 20.3% (risk ratio 2.279, 95% CI, 1.632–3.182). Early recognition of oesophageal perforation is difficult due to the non-specific nature of symptoms. Therefore, a high index of suspicion is fundamental, especially after any oesophageal instrumentation or intra-thoracic surgery.
Timing of diagnosis, the presence of systemic symptoms, the site and size of the perforation, patient’s comorbidities and underlying oesophageal pathology (9,10) affects approaches and outcomes. Surgery has traditionally been the mainstay of treatment, with options ranging from primary repair and thorough drainage of the mediastinum and pleural spaces (11) to exclusion and diversion techniques, to oesophagectomy (1,12). Surgery remains challenging even in very experienced hands and reported morbidity and failure rates are high (1,13), particularly in patients with delayed diagnosis (11). The last couple of decades have seen an increasing popularity of diversified non-surgical management options, such as endoscopic clipping (14), suturing (15) and stenting (16,17), originally in patients not suitable for surgery, and later as an alternative first choice treatment option in a selected group (18-20). Novel approaches such as endoscopic vacuum-assisted therapy (EVT) have become available in recent years with encouraging results (21-25).
The present review outlines the current approach to iatrogenic oesophageal perforation. Spontaneous perforations as well as oesophageal anastomotic leaks or fistulae after surgery are not part of this analysis.
Aetiology
Iatrogenic causes of oesophageal perforation are the most common (2,3,10,26) with endoscopic interventions associated with the highest number of cases (27-29). Table 1 summarizes different causes of iatrogenic oesophageal perforation.
Table 1
| Intubation of the oesophagus (rigid and flexible) |
| Nasogastric tube |
| Diagnostic endoscopy |
| Interventional endoscopy (biopsy, dilatation, laser therapy, photodynamic therapy, variceal sclerotherapy, endomucosal resection, peroral endoscopic myotomy, stent placement/removal, foreign body removal, ERCP*) |
| Transoesophageal ultrasound (transoesophageal echocardiography, endoscopic ultrasound of the oesophagus and/or fine needle aspiration) |
| Intubation of the trachea |
| Endotracheal intubation, tracheostomy, minitracheostomy, bronchoscopy, EBUS** |
| Oesophageal surgery |
| Heller myotomy, vagotomy, hiatal hernia repair, anti-reflux surgery, duplication cyst resection, diverticulum surgery, enucleation of tumours of the oesophageal wall, oesophagectomy |
| Non-oesophageal surgery |
| Thoracic (mediastinoscopy, intrathoracic lymphadenectomy, airway surgery, pneumonectomy, lung transplantation, atrial surgery, aortic surgery, resection of mediastinal tumours) |
| Cervical (thyroid, spinal, vascular surgery) |
*ERCP, endoscopic retrograde cholangiopancreatography; **EBUS, endobronchial ultrasound.
The adoption of endoscopic screening programmes and the advancements in therapeutic endoscopy have increased the number of patients undergoing endoscopic procedures. Although any intubation of the oesophagus can potentially cause a perforation, the European Society of Gastrointestinal Endoscopy (ESGE) suggests that, of all endoscopic procedures, dilatations, mucosal resections or submucosal dissections, and the removal of foreign bodies are amongst the riskiest procedures in this respect (20).
Endotracheal intubation (31,32) particularly if complex or emergent, can cause oesophageal perforation, although rarely.
Intraoperative oesophageal perforation is far less common, and can be associated with oesophageal or non-oesophageal surgery.
With respect to non-oesophageal surgery, the procedures associated with a risk of oesophageal perforation are those where a dissection of the neck or posterior mediastinum are involved, such as cervical spine surgery (33), mediastinoscopy (34), nodal dissection for lung cancer or airway surgery (35). However, there is limited published data on the rate of perforation in these circumstances. Without a high index of suspicion, such complications might not be diagnosed intraoperatively or even early postoperatively. Occasionally, the delayed appearance of oesophageal perforation after complex extensive dissections of the mediastinum has been put down to devascularization of the oesophagus as the result of ligation of multiple bronchial and oesophageal arteries (36).
Presentation
The presentation of iatrogenic oesophageal perforation depends on site of perforation and timing of injury. Pain is the most common symptom, present in about 70% of cases. The acute onset of pain (29) after an endoscopic procedure is probably the most common clinical scenario. The location of pain will typically reflect the site of perforation. Other clinical features include dysphagia, dyspnoea, nausea and vomiting, fever, tachycardia and tachypnoea (1).
An oesophageal perforation after surgery can be diagnosed with significant delay as the use of strong painkillers will mitigate the pain and all other signs might appear as relatively mild or non-specific. A high index of suspicion for perforation should exist in patients presenting with any combination of the above-mentioned clinical features after oesophageal instrumentation or intra-thoracic surgery (1).
Cervical perforation of the oesophagus can result in neck pain, stiffness during flexion and subcutaneous emphysema. The development of a systemic inflammatory response or sepsis can be slow due to anatomical containment by the neck fascial planes (1).
Thoracic oesophageal perforation results in the extravasation of saliva, food bolus, and refluxed gastric content into the mediastinum and, subsequently, the pleural spaces. The evolution into severe sepsis with multiorgan failure is quick. Pneumomediastinum will usually be evident on imaging, particularly in the posterior mediastinum. As the mediastinal pleura is breached, unilateral or bilateral empyemas with pneumothorax will appear.
Perforation of the intrabdominal oesophagus may present with abdominal pain or referred pain to the shoulder due to diaphragmatic irritation. Uncontained perforations quickly evolve into peritonitis and systemic sepsis.
Diagnosis
Diagnosis of oesophageal perforation is a recognised challenge. It is estimated that only 58% of oesophageal perforations from all aetiologies will be admitted and treated within 24 hours (3).
Immediate referral for appropriate imaging is mandatory in any patients after endoscopic or surgical intervention where perforation is suspected.
Imaging modalities include plain radiology and contrast imaging. Plain radiology will show indirect signs of oesophageal perforation such as pleural effusions, pneumothorax, pneumomediastinum, pneumopericardium, subcutaneous emphysema or free-air under the diaphragm in up to 90% of patients (1).
Computed tomography (CT) scanning is commonly used and readily accessible in the assessment of acutely unwell patients. It is more sensitive than X-rays in showing small collections of air or fluid in the mediastinum or peritoneum, hence ESGE (20) recommends CT as the go-to investigation in order to avoid diagnostic delay. Soluble contrast imaging will demonstrate the actual perforation. Again, oral contrast CT will be more accurate and informative than fluoroscopy, particularly with respect to demonstrating small leaks, showing small mediastinal collections and offering guidance to place percutaneous drains or to plan surgery (37). A risk of aspiration of soluble contrast and consequent necrotizing pneumonia in severely ill patients must be taken into account (1,27). The adoption of ad hoc optimized CT protocols for suspected oesophageal perforation (including lower neck, chest and upper abdomen and scanning both pre- and post-oral contrast administration) can greatly improve the accuracy and efficiency of the diagnostic work-up with a one-stop-shop tool (38).
Traditionally, the role of flexible endoscopy in the diagnosis of oesophageal perforation has been controversial, mainly due to concerns that gas insufflation might increase the extraluminal contamination. Such concerns have not been conclusively demonstrated in the literature and the ESGE recommends that they should not delay the diagnosis or treatment of iatrogenic oesophageal perforations (20). Moreover, the wider utilization of endoscopy in the workup and treatment of oesophageal perforations in the last couple of decades reinforces the argument that endoscopy is safe and plays an important role in the management of acute oesophageal perforations (20).
Treatment
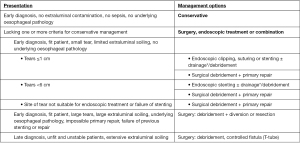
The treatment of iatrogenic oesophageal perforations follows either conservative, endoscopic or surgical approach (Figure 1). This will depend on aetiology, site of perforation and extent of contamination as well as presence of any underlying oesophageal disease (1,3,4,27,39). Various authors have proposed treatment algorithms to rationalize and expedite decision making (1,13,28,39). Ultimately, though, none of these are evidence based as there are no prospective randomized trials, or indeed well-designed comparative studies, to support one treatment option or the other. Therefore, the treatment of iatrogenic oesophageal perforation remains largely controversial.
Regardless of therapeutic approach, longer time to treatment negatively affects outcome (1,3,8,28), with mortality roughly ranging from around 15%, if the perforation is treated within 24 hours, to 40% if treated after more than 24 hours.
Conservative treatment
The main elements of conservative management for oesophageal perforation are fluid resuscitation, intravenous antimicrobials, nil by mouth, gastric decompression, nutritional support, close haemodynamic monitoring and support, percutaneous drainage of collections and analgesia. These elements still hold true as basic principles to support (and overlap with) both the endoscopic and surgical treatment.
The selection criteria for an upfront purely conservative approach are narrow and controversial, but generally agreed as early diagnosis (<24 hours), no extraluminal contamination, absence of sepsis or oesophageal pathology that might prevent spontaneous healing (such as persistent obstruction, malignancy or end-stage benign disease) (27,40,41).
Conservative treatment is generally most successful in iatrogenic perforations of the cervical oesophagus, as they are more likely to match the above-mentioned selection criteria, although some small retrospective cohorts showed good results in intra-thoracic perforations too (42-44). Close imaging monitoring with CT scans and oesophagogram, along with early percutaneous drainage of any extraluminal collection is advocated to achieve good outcomes (42), as inadequate control of the mediastinal sepsis remains the main cause of the high mortality rate in patients with delayed operative treatment. Survival rates of 84.6% to 100% have been reported in the most successful series (42-44) in other series, however, later operative management was required in 20% (41) to 41% (45).
Surgical treatment
Surgery has historically been the mainstay of treatment in oesophageal perforations. Indications and techniques are based on retrospective series and expert opinion.
As with other treatment options, the surgical approach will depend on characteristics of the perforation, degree of contamination and sepsis, clinical status of the patient, nature and extent of any underlying oesophageal pathology, and surgeon’s experience.
Thorough debridement and drainage of contaminated tissue around the perforation and primary repair has been the preferred surgical option in fit patients with early diagnosis and otherwise healthy oesophagus. Reinforcing the repair with well vascularized flaps is recommended to reduce postoperative leaks, particularly if repair is delayed more than 24 hours (11,27,46,47). Any underlying oesophageal pathology that might preclude healing, such as the presence of a concomitant obstruction, must be addressed at the time of repair. Even with adequate surgical repair, post-operative leaks are reported in about 30% of patients and up to 40% will need additional procedures (13).
Where primary repair is not possible due to extensive oesophageal damage, diversion and exclusion techniques have been used. In severely ill, unstable patients, surgery is limited to draining the mediastinum and creating a controlled fistula with a T-tube. Unsurprisingly, these approaches are associated with higher morbidity and mortality and are becoming less common (4,28).
Large circumferential perforations, non-dilatable strictures, early stage malignancy and end stage benign disease represent indications for resection and reconstruction (27,28,47). Oesophagectomy has also been used where primary repair or stenting have failed to control sepsis (1,9).
Brinster et al. (1) report a pooled mortality rate of 17% for resection of all acute oesophageal perforations (on a systematic review including 129 patients between 1990 and 2003), whilst primary repair had a mortality of 12% (on 322 patients) and exclusion/diversion had a mortality of 24% (on 31 patients). In a metanalysis of 75 studies (2,971 patients) published in 2013, Biancari et al. (8) reported a pooled mortality of 13.2% for all acute oesophageal perforations. Mortality was 9.5% in the primary repair population (7.4% if repair within 24 hours and 29% if repair after 24 hours), 13.8% in the oesophagectomy group and 20% in the exclusion/diversion group.
Seo et al. (12) published a notably low 30-day mortality of 9% and 5% in a retrospective series of 90 emergent esophagectomies for acute perforation with underlying benign or malignant disease, respectively.
Bhatia et al. (4) performed a multivariate logistic regression analysis on 119 oesophageal perforations (51% iatrogenic) between 1981 and 2007 and found that, whilst time to treatment is associated with mortality, the presence of malignancy and clinical status at the time of diagnosis—such as mechanical ventilation, pulmonary comorbidity and higher overall comorbidity burden—are greater predictors of mortality.
Endoscopic treatment
Historically, endoscopic approaches to iatrogenic oesophageal perforations were reserved for managing inveterate fistulae, perforation of inoperable malignancies, patients unfit for surgery or after the failure of surgical repair. Since the early 2000s, the advent of new generations of removable occlusive stents (48) has opened the doors to the utilization of stents to achieve temporary occlusion of the perforation until oesophageal healing occurs.
A few years later, Freeman (17) reported the first cohort of 17 patients that were offered stenting instead of surgery as first treatment option for intrathoracic iatrogenic perforations. It was, in fact, a hybrid approach, as thoracoscopic drainage of pleural spaces and mediastinum was performed at the same time of the stent when indicated. Results were encouraging, with 82% of patients resuming nutrition within 72 hours, 94% success in healing the perforation, and no mortality. Migration of the stent requiring a repeat procedure occurred in 18% of patients. This approach is reproducible in experienced hands and with adequate patient selection, as a number of small endoscopic stenting series have been published with similar results; (9,13,26,49-53) mortality rates of 0% to 14% (with better outcomes for early intervention), reintervention of 0–38%, migration of stents 13–40%, and success rates of 62–100%. It appears that the optimum timing for subsequent stent removal is around 2 weeks (49).
Limitations of the stenting approach include perforations located in the proximal cervical oesophagus or gastroesophageal junction, and defects longer than 6 cm, where failure (persistent leak or migration) is more likely (54).
Endoscopic clips have been successfully used with good outcomes for patients with small defects caused during endoscopic instrumentation, using a combination of through the scope and over the scope clips (14,18). Successful placement requires viable surrounding tissue.
The ESGE advocates the use of endoscopic clips or suturing (15) in stable patients with early diagnosis and a small (<10 mm) contained perforation, with higher rates of success in the cervical oesophagus. It recommends stenting for larger perforations, although it states “it is unlikely that holes larger than 3 cm may be endoscopically treated” (20).
More novel therapies include the use of EVT which was initially described in the management of anastomotic leaks (55). Vacuum therapy encourages healing by secondary intention with the formation of granulation tissue and removal of contaminating fluid. Direct visualisation of the perforation site and cavity is done via endoscopy and a sponge placed into the site of injury via a nasogastric tube through which suction is applied. Endoscopic lavage can be used. Monitoring and subsequent endo-sponge replacement is done with repeated endoscopies, initially every 3–5 days (25). A number of small studies are published on the use of EVT in acute perforations (21-24). In a series of 10 patients with iatrogenic perforations secondary to endoscopy, all patients were diagnosed in less than 24 hours and successfully managed with EVT (24). Another small series of 10 patients with a broader range of causes (23) (iatrogenic, spontaneous) reported complications in 70%, including mediastinitis, pleural empyema and sepsis. They required further intervention in form of chest drainage and one required a video-assisted thoracoscopic (VATS) washout and decortication. Reported mortality was 10% across all causes. In comparison to established interventional options, patients are required to remain nothing-by-mouth for the duration of EVT and some studies report longer hospital stays (23).
The great variability in the anatomical and clinical presentation of iatrogenic oesophageal perforations, severity of patient’s condition and comorbidities, time to treatment, availability of local diagnostic and therapeutic skills, explains why there is such a diversification of available approaches to management.
Moreover, the available literature is inconclusive and unable to indicate a superior treatment option, as based mostly on small, single-institution, retrospective series, with the inherent flaws and biases of the reported outcomes and the lack of adequate comparisons between the different therapeutic strategies.
In his systematic review of the literature 2005–2015, Sdralis et al. (3) reports that, out of 52 eligible papers, 43 were of ‘low’ or ‘very low’ quality according to GRADE classification of Cochrane handbook (56).
As iatrogenic oesophageal perforation remains an uncommon complication requiring multidisciplinary competencies, unsurprisingly, better outcomes are reported in high volume tertiary centres (2,20).
Over the last couple of decades, although basic principles of management have stayed the same (stopping extraluminal soilage, preventing sepsis, re-establishing enteral continuity, close monitoring and haemodynamic support and nutrition), the therapeutic strategies have moved from an absolute dominion of “surgery whenever feasible” to a more conservative, tailored and dynamic approach that follows the patient’s presentation and clinical evolution (19), often ending with successful hybrid solutions (e.g., conservative combined with stents and VATS washout of empyema) (17,18). Markar et al. (2) noticed a reduction in surgical management in favour of conservative/endoscopic approach in their review of all oesophageal perforations recorded in England over the study period 2000–2012.
Conclusions
Iatrogenic oesophageal perforation remains an uncommon but potentially devastating injury associated with high morbidity and mortality. Early diagnosis is vital but difficult and only about half of the patients receive treatment within the first 24 hours.
The management has transitioned over the years towards a multidisciplinary approach where radiological, endoscopic and surgical competencies are all required to be readily available. A successful outcome depends on careful patient selection as well as timing and efficacy of diagnostic and therapeutic choices. However, a well-defined optimal strategy is inexistent and the lack of evidence and reproducible data to guide patient selection and inform decision making is partly responsible for the still suboptimal outcomes of iatrogenic oesophageal perforations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (David Waller) for the series “Complications of Thoracic Surgery – aetiology, management and prevention” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form, (available at: http://dx.doi.org/10.21037/shc-21-10). The series “Complications of Thoracic Surgery – aetiology, management and prevention” was commissioned by the editorial office without any funding or sponsorship. Both authors have no other conflicts of interest to declare.
Ethical Statement: Both authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475-83. [Crossref] [PubMed]
- Markar SR, Mackenzie H, Wiggins T, et al. Management and Outcomes of Esophageal Perforation: A National Study of 2,564 Patients in England. Am J Gastroenterol 2015;110:1559-66. [Crossref] [PubMed]
- Sdralis EIK, Petousis S, Rashid F, et al. Epidemiology, diagnosis, and management of esophageal perforations: systematic review. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Bhatia P, Fortin D, Inculet RI, et al. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg 2011;92:209-15. [Crossref] [PubMed]
- Bayram AS, Erol MM, Melek H, et al. The success of surgery in the first 24 hours in patients with esophageal perforation. Eurasian J Med 2015;47:41-7. [Crossref] [PubMed]
- Shaker H, Elsayed H, Whittle I, et al. The influence of the 'golden 24-h rule' on the prognosis of oesophageal perforation in the modern era. Eur J Cardiothorac Surg 2010;38:216-22. [Crossref] [PubMed]
- Eroglu A, Can Kürkçüogu I, Karaoganogu N, et al. Esophageal perforation: the importance of early diagnosis and primary repair. Dis Esophagus 2004;17:91-4. [Crossref] [PubMed]
- Biancari F, D'Andrea V, Paone R, et al. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg 2013;37:1051-9. [Crossref] [PubMed]
- Law TT, Chan JY, Chan DK, et al. Outcomes after oesophageal perforation: a retrospective cohort study of patients with different aetiologies. Hong Kong Med J 2017;23:231-8. [Crossref] [PubMed]
- Wahed S, Dent B, Jones R, et al. Spectrum of oesophageal perforations and their influence on management. Br J Surg 2014;101:e156-62. [Crossref] [PubMed]
- Wright CD, Mathisen DJ, Wain JC, et al. Reinforced primary repair of thoracic esophageal perforation. Ann Thorac Surg 1995;60:245-8; discussion 248-9. [Crossref] [PubMed]
- Seo YD, Lin J, Chang AC, et al. Emergent Esophagectomy for Esophageal Perforations: A Safe Option. Ann Thorac Surg 2015;100:905-9. [Crossref] [PubMed]
- Kiev J, Amendola M, Bouhaidar D, et al. A management algorithm for esophageal perforation. Am J Surg 2007;194:103-6. [Crossref] [PubMed]
- Lázár G, Paszt A, Mán E. Role of endoscopic clipping in the treatment of oesophageal perforations. World J Gastrointest Endosc 2016;8:13-22. [Crossref] [PubMed]
- Gaur P, Lyons C, Malik TM, et al. Endoluminal suturing of an anastomotic leak. Ann Thorac Surg 2015;99:1430-2. [Crossref] [PubMed]
- Fischer A, Thomusch O, Benz S, et al. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg 2006;81:467-72. [Crossref] [PubMed]
- Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg 2007;83:2003-7; discussion 2007-8. [Crossref] [PubMed]
- Dickinson KJ, Blackmon SH. Endoscopic Techniques for the Management of Esophageal Perforation. Oper Tech Thorac Cardiovasc Surg 2015;20:251-78. [Crossref]
- Sudarshan M, Elharram M, Spicer J, et al. Management of esophageal perforation in the endoscopic era: Is operative repair still relevant? Surgery 2016;160:1104-10. [Crossref] [PubMed]
- Paspatis GA, Dumonceau JM, Barthet M, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2014;46:693-711. [Crossref] [PubMed]
- Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 2016;30:2473-80. [Crossref] [PubMed]
- Leeds SG, Burdick JS, Fleshman JW. Endoluminal Vacuum Therapy for Esophageal and Upper Intestinal Anastomotic Leaks. JAMA Surg 2016;151:573-4. [Crossref] [PubMed]
- Heits N, Stapel L, Reichert B, et al. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 2014;97:1029-35. [Crossref] [PubMed]
- Loske G, Schorsch T, Dahm C, et al. Iatrogenic perforation of esophagus successfully treated with Endoscopic Vacuum Therapy (EVT). Endosc Int Open 2015;3:E547-51. [Crossref] [PubMed]
- de Moura DTH, de Moura BFBH, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc 2019;11:329-44. [Crossref] [PubMed]
- Lindenmann J, Matzi V, Neuboeck N, et al. Management of esophageal perforation in 120 consecutive patients: clinical impact of a structured treatment algorithm. J Gastrointest Surg 2013;17:1036-43. [Crossref] [PubMed]
- Kaman L, Iqbal J, Kundil B, et al. Management of Esophageal Perforation in Adults. Gastroenterology Res 2010;3:235-44. [PubMed]
- Lampridis S, Mitsos S, Hayward M, et al. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J Thorac Dis 2020;12:2724-34. [Crossref] [PubMed]
- Nirula R. Esophageal perforation. Surg Clin North Am 2014;94:35-41. [Crossref] [PubMed]
- Tournoy KG, Burgers SA, Annema JT, et al. Transesophageal endoscopic ultrasound with fine needle aspiration in the preoperative staging of malignant pleural mesothelioma. Clin Cancer Res 2008;14:6259-63. [Crossref] [PubMed]
- Jougon J, Cantini O, Delcambre F, et al. Esophageal perforation: life threatening complication of endotracheal intubation. Eur J Cardiothorac Surg 2001;20:7-10; discussion 10-1. [Crossref] [PubMed]
- Hilmi IA, Sullivan E, Quinlan J, et al. Esophageal tear: an unusual complication after difficult endotracheal intubation. Anesth Analg 2003;97:911-4. [Crossref] [PubMed]
- Halani SH, Baum GR, Riley JP, et al. Esophageal perforation after anterior cervical spine surgery: a systematic review of the literature. J Neurosurg Spine 2016;25:285-91. [Crossref] [PubMed]
- Weiss AJ, Salter B, Evans A, et al. Esophageal perforation following cervical mediastinoscopy: a rare serious complication. J Thorac Dis 2015;7:E678-81. [PubMed]
- Amer K, Khan AZ, Parshad R, et al. VATS nodal dissection: caveats, complications and troubleshooting [video recording]. Asvide AME Surgical Video Database. AME Publishing Company. 2018. Available online: https:// www.asvide.com/article/view/25401
- Venuta F, Rendina EA, De Giacomo T, et al. Esophageal perforation after sequential double-lung transplantation. Chest 2000;117:285-7. [Crossref] [PubMed]
- Upponi S, Ganeshan A, D'Costa H, et al. Radiological detection of post-oesophagectomy anastomotic leak - a comparison between multidetector CT and fluoroscopy. Br J Radiol 2008;81:545-8. [Crossref] [PubMed]
- Madan R, Laur O, Crudup B, et al. Imaging of iatrogenic oesophageal injuries using optimized CT oesophageal leak protocol: pearls and pitfalls. Br J Radiol 2018;91:20170629 [PubMed]
- Minnich DJ, Yu P, Bryant AS, et al. Management of thoracic esophageal perforations. Eur J Cardiothorac Surg 2011;40:931-7. [PubMed]
- Shaffer HA, Valenzuela G, Mittal RK. Esophageal perforation. A reassessment of the criteria for choosing medical or surgical therapy. Arch Intern Med 1992;152:757-61. [Crossref] [PubMed]
- Altorjay A, Kiss J, Vörös A, et al. Nonoperative management of esophageal perforations. Is it justified? Ann Surg 1997;225:415-21. [Crossref] [PubMed]
- Vogel SB, Rout WR, Martin TD, et al. Esophageal perforation in adults: aggressive, conservative treatment lowers morbidity and mortality. Ann Surg 2005;241:1016-21; discussion 1021-3. [Crossref] [PubMed]
- Hasan S, Jilaihawi AN, Prakash D. Conservative management of iatrogenic oesophageal perforations--a viable option. Eur J Cardiothorac Surg 2005;28:7-10. [Crossref] [PubMed]
- Mao JC, Kayali FM, Dworkin JP, et al. Conservative management of iatrogenic esophageal perforation in head and neck cancer patients with esophageal stricture. Otolaryngol Head Neck Surg 2009;140:505-11. [Crossref] [PubMed]
- Amir AI, van Dullemen H, Plukker JT. Selective approach in the treatment of esophageal perforations. Scand J Gastroenterol 2004;39:418-22. [Crossref] [PubMed]
- Sung SW, Park JJ, Kim YT, et al. Surgery in thoracic esophageal perforation: primary repair is feasible. Dis Esophagus 2002;15:204-9. [Crossref] [PubMed]
- Bufkin BL, Miller JI, Mansour KA. Esophageal perforation: emphasis on management. Ann Thorac Surg 1996;61:1447-51; discussion 1451-2. [Crossref] [PubMed]
- Herrera A, Freeman RK. The Evolution and Current Utility of Esophageal Stent Placement for the Treatment of Acute Esophageal Perforation. Thorac Surg Clin 2016;26:305-14. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Dake M, et al. An Assessment of the Optimal Time for Removal of Esophageal Stents Used in the Treatment of an Esophageal Anastomotic Leak or Perforation. Ann Thorac Surg 2015;100:422-8. [Crossref] [PubMed]
- Navaneethan U, Lourdusamy V, Duvuru S, et al. Timing of esophageal stent placement and outcomes in patients with esophageal perforation: a single-center experience. Surg Endosc 2015;29:700-7. [Crossref] [PubMed]
- Suzuki T, Siddiqui A, Taylor LJ, et al. Clinical Outcomes, Efficacy, and Adverse Events in Patients Undergoing Esophageal Stent Placement for Benign Indications: A Large Multicenter Study. J Clin Gastroenterol 2016;50:373-8. [Crossref] [PubMed]
- Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esophagus 2005;18:262-6. [Crossref] [PubMed]
- Persson S, Elbe P, Rouvelas I, et al. Predictors for failure of stent treatment for benign esophageal perforations - a single center 10-year experience. World J Gastroenterol 2014;20:10613-9. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Giannini T, et al. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg 2012;94:959-64; discussion 964-5. [Crossref] [PubMed]
- Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 2014;20:7767-76. [Crossref] [PubMed]
- Schünemann HJ, Higgins JPT, Vist GE, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.
0. 2019. Cochrane . Available online www.training.cochrane.org/handbook
Cite this article as: Rozwadowski S, Internullo E. Iatrogenic oesophageal perforation. Shanghai Chest 2021;5:37.