
Bronchoscopic lung volume reduction: who, what, where, when?
Introduction
Chronic obstructive pulmonary disease (COPD) affects over 10% of the world’s population (1). It is the third leading cause of mortality worldwide, and the number of affected individuals continues to increase due to continued exposure to risk factors and an aging population (1,2). In 2012, more than 3 million people died from COPD, accounting for 6% of all deaths worldwide (2). Hospitalizations are predicted to increase by >150% over the next 15 years in developed countries, while the number of people with COPD over age 75 years of age is predicted to increase by 220% (3). It is a progressive condition, most commonly caused by cigarette smoking. Other etiologies include genetic conditions (alpha-1 antitrypsin deficiency) and environmental (biomass fuel, air pollution, etc.) and occupational exposures in approximately 10% of cases (4). Symptoms include cough and dyspnea, resulting in reduced exercise capacity.
Treatment is stage dependent and has historically included bronchodilators, pulmonary rehabilitation, and smoking cessation (5). Adherence to therapy has been demonstrated to improve surrogate outcome measures, including number of exacerbations, spirometry, and quality of life (QoL) (6). Bronchodilators include long-acting muscarinic antagonists, long-acting beta agonists, and inhaled corticosteroids. Refractory cases may also utilize chronic oral corticosteroids, chronic suppressive antibiotics, phosphodiesterase inhibitors, and supplemental oxygen (7). For patients with emphysema, options beyond medical management have historically been limited to very select patients and have involved surgical treatment with either lung volume reduction surgery (LVRS) or lung transplantation (8). The National Emphysema Treatment Trial (NETT) demonstrated improved pulmonary function, exercise capacity, and QoL with LVRS in patients with upper lobe predominant emphysema and poor baseline exercise capacity (9,10). High morbidity, mortality, and costs have hindered its widespread adoption (11-13).
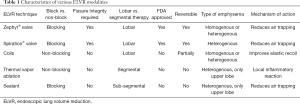
Since the introduction of LVRS, various endoscopic lung volume reduction (ELVR) modalities have been developed (Table 1). ELVR options commercially available globally include valves, coils, vapor, and sealant (14). Of these, only valves are approved for use in the United States (US). Broadly, these techniques can be classified as “block” [endobronchial valve (EBV), sealant] and “non-block” (coils, vapor) (15). The same groupings also apply to their reversibility, except for sealant. Valves include the Zephyr® Endobronchial Valve (Pulmonx, Redwood City, CA, USA) and the Spiration® Valve System (SVS) (Olympus, Tokyo, Japan). EBVs have now been incorporated into guidelines, including those from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the National Institute for Health and Care Excellence (NICE) (16,17). They are also approved by the US Food and Drug Administration (FDA) since 2018 (18,19). Attention will be focused on EBVs for the remainder of the discussion since they are the only ELVR modality currently in widespread use outside of clinical trials.
Full table
Physiologic rationale
A subset of patients with COPD develop emphysematous destruction of parenchymal tissue due to chronic inflammation. In turn, this causes permanent enlargement of the terminal bronchioles, which leads to impaired gas exchange, dynamic hyperinflation, loss of elastic recoil, air trapping, and increased residual volume (RV) (20). Hyperinflation impairs normal respiratory muscle mechanics and decreases chest wall compliance (21). As work of breathing increases, exercise tolerance decreases, creating a vicious cycle. While traditional pharmacologic therapy for COPD decreases respiratory symptoms and improves exercise capacity, it does not reverse the underlying pathophysiology or the trajectory of deteriorating respiratory function.
LVRS is based on the principle of resecting the most damaged lobe in patients with heterogeneous upper lobe predominant emphysema, thereby reducing hyperinflation. This reduction decreases pressure on the chest wall and respiratory muscles, including the diaphragm, allowing them to assume a more natural conformation and hence function more effectively.
EBVs have been designed to achieve the same reduction in hyperinflation as LVRS but with less morbidity and mortality. They are intended to produce complete occlusion of the segmental or subsegmental airways in a lobe, which should lead to lobar atelectasis if there is no collateral ventilation (CV). At least a 350 mL reduction in volume is necessary for patients to appreciate a clinical benefit (22). Identification of CV is discussed in more detail in the section on patient selection.
Endobronchial valves
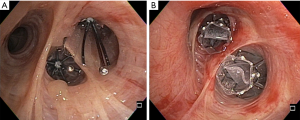
The Spiration® and Zephyr® valves differ in their structure and composition, as seen in Figure 1. While both are built using a nitinol (combination of nickel and titanium) framework, the Spiration® valve has an umbrella-like shape and utilizes a polyurethane covering (Table 2). The Zephyr® valve is duckbill shaped and employs a silicone covering. Although structurally unique, both valves are designed to achieve the same outcome—lobar atelectasis via complete one-way occlusion of the airway. In order to achieve this result, CV must be absent. Both valves allow unidirectional flow in the proximal direction during expiration, so that the treated lobe is vented (23). This allows atelectasis to develop and produce the desired volume-reducing effect.

Full table
Different techniques are required to measure airway diameter and ensure appropriate fit. The SVS uses a balloon catheter that must be carefully calibrated preoperatively, while the Zephyr® valve relies on two sets of small wings attached to the distal aspect of the deployment catheter. These wings approximate the diameter of the two valve sizes. After measuring the airway diameter, they are deployed through a flexible bronchoscope using a catheter inserted via the working channel. After positioning the catheter appropriately, the sheath is retracted, and the EBV springs open and into position. Because precise positioning is required, these procedures are most often performed under general anesthesia in an endoscopy suite or operating room. Radial force against the airway wall limits migration with the Zephyr® valve, while anchors help maintain position of the Spiration® valve.
Patient selection
Candidates for EBVs must meet a number of criteria. First and foremost, they must have emphysema with hyperinflation and be highly symptomatic despite receiving optimal pharmacologic management, including bronchodilators, inhaled corticosteroids, and systemic therapy if necessary. Smoking cessation for at least 4 months is mandatory. They should have completed or be actively enrolled in a formal pulmonary rehabilitation program or comparable structured physical therapy program. Nutritional status should be optimized with the help of a dietician. Chronic hypoxic respiratory failure necessitating supplemental oxygen at home is not an absolute contraindication. Contraindications include inability to tolerate a bronchoscopic procedure, active pulmonary infection, active smoking, allergy to the device material, and large bullae occupying greater than one-third of the lung (18,19). In the clinical trials, patients with a mean pulmonary artery systolic pressure (PASP) >45 mmHg and/or with a PaCO2 >50 mmHg were excluded (24,25).
The medical evaluation consists of complete pulmonary function testing (spirometry with a bronchodilator, diffusion capacity, and lung volumes obtained via body plethysmography), a volumetric high-resolution computed tomography (HRCT) scan, a 6-minute walk test (6MWT), a transthoracic echocardiogram (TTE), and an arterial blood gas (ABG) on room air (Table 3). Acceptable values for this testing based on LIBERATE and EMPROVE trial criteria are delineated in Table 4 (26). A right heart catheterization (RHC) may be required to rule out pulmonary hypertension (PH) when the TTE suggests a right ventricular systolic pressure (RVSP) >50 mmHg.

Full table

Full table
Computer analysis of the HRCT quantifies the degree of emphysematous destruction by lobe and fissure completeness, which is thought to correspond with inter-lobar CV. Utilization of this quantitative imaging software is recommended by an expert panel (26). Reports for the two approved EBVs have slight differences. The SeleCT® Report used for the SVS provides a calculated heterogeneity score. The StratX® Report used for the Zephyr valve provides emphysema destruction scores for threshold values of both –910 and –950 Hounsfield units (HU). It also provides a combined measurement for the right upper and middle lobes, in addition to the right upper lobe individually.
The more complete the fissure, the less likely there is to be CV. The ideal HRCT protocol involves a non-contrast scan on a multi-detector platform with thin (0.6–1.25 mm) slices with some overlap and smooth kernel reconstructions. The exact protocol depends on the manufacturer of the HRCT equipment. Incidental findings, including bronchiectasis, pulmonary nodules, and interstitial fibrosis should be addressed prior to proceeding with ELVR. A threshold value of >40–50% destruction (percentage of voxels <–910 HU) is utilized when identifying an acceptable target lobe (24,25). Heterogenous emphysema is typically defined as an absolute difference ≥10–15% in the destruction scores between the target lobe and the ipsilateral lobe(s) (24,25,27).
When there are two or more acceptable target lobes based on computer analysis of the HRCT, a nuclear medicine single photon emission tomography computed tomography (NM SPECT CT) scan may be performed to quantify the degree of ventilation and perfusion by lobe of the lungs, in order to identify the optimal target lobe. Among the potential target lobes, the one with the least perfusion should be selected preferentially in order to minimize the amount of ventilation/perfusion (V/Q) mismatching that occurs after lobar occlusion with EBVs (28).
With StratX® analysis software (used for Zephyr® valves), fissure completeness scores (FCSs) of ≥95% and ≤80% are defined as complete and incomplete, respectively. Scores in the 80–95% range are defined as partially complete. In this subgroup of patients, Chartis® (Pulmonx Inc., Redwood City, CA, USA) assessment is recommended to assist with identifying the presence of CV and has an overall accuracy of 83.3% (26,29). The Chartis® system is a balloon catheter that measures pressure, flow, and resistance after balloon occlusion of a lobar bronchus. Lack of CV is confirmed when the air flow decreases to zero after occlusion of an airway. Chartis® assessment is optional above 95% fissure completeness and unnecessary <80%, since these latter patients are not candidates for EBVs. Chartis® assessment can be safely and accurately performed under either moderate sedation or general anesthesia (22). Early studies suggested that together, computer analysis of the HRCT and the Chartis® assessment have a pooled sensitivity of 75% for detecting CV (30). Recent data have shown that for the left and right major fissures, a FCS >95% has a sensitivity of 91.1% and 73.7%, respectively (31). Thus, Chartis® assessment is recommended on the right regardless of quantitative CT fissure analysis.
There are no data using the Chartis® system with Spiration® valves. CT scan analysis using different software was performed in the EMPROVE trial, where a ≥90% fissure integrity threshold was utilized (24). This software differs in that no combined assessment of the right upper lobe and right middle lobe is provided. Heterogeneity is also reported. For reference, the EMPROVE thresholds are listed on the report, and values meeting those criteria are highlighted.
Management of complications
EBVs have rapidly gained popularity because of the low morbidity and mortality rate associated with this therapy. Complications, however, still occur and must be addressed. A recent meta-analysis identified the following risk ratios (RRs) (32):
- Pneumothorax RR: 9.65 (3.04–30.6);
- Mild hemoptysis RR: 6.42 (1.21–34.01);
- Hospitalization for a COPD exacerbation RR: 2.01 (1.19–3.40).
These adverse events were not associated with a significant risk of death [RR: 1.56 (0.47–5.18)] or pneumonia [RR: 2.17 (0.86–5.49)].
The most common adverse event is pneumothorax, which is more likely with complete lobar atelectasis, especially when it occurs rapidly. Pneumothorax occurs because of compensatory over-expansion of the ipsilateral untreated lobe(s) (33). In both the EMPROVE and LIBERATE trials, the majority of pneumothoraces occurred within the first 72 hours, so close observation in the hospital is recommended for at least 3 days postoperatively (24-26). Tube thoracostomy drainage alone is usually sufficient. If drainage is not performed in an expeditious manner, tension pneumothorax may develop and could be fatal. A management algorithm has been developed by Valipour et al. for refractory cases (34). If a persistent air leak (PAL) continues beyond 7 days, then removal of one EBV is recommended. If the PAL still continues for 48 hours, then remove the remaining valves. If the PAL still fails to resolve, then consider pleurodesis or surgical intervention. There is no role for prophylactic tube thoracostomy intraoperatively.
There is no role for preoperative steroids. Some authors also recommend steroids and prophylactic antibiotic therapy for 1 week after ELVR to reduce inflammation and the risk of a COPD exacerbation or pneumonia (23). Limited data are available to support this practice.
Evidence
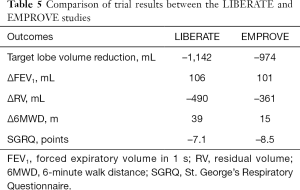
A number of randomized controlled trials (RCTs) have been conducted with both types of EBVs in order to demonstrate safety and efficacy. Key findings from the trials that led to US FDA approval are summarized below and presented in Table 5.
Full table
Zephyr® valve
The multicenter RCT of Zephyr® Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE) trial randomized 190 subjects in a 2:1 ratio to treatment with EBV and standard of care (SoC) at 24 sites (25). At 12 months, forced expiratory volume in 1 s (FEV1) improved ≥15% in 47.7% and 16.8% in the EBV group and SoC group, respectively (P<0.001). The absolute improvement in the EBV group versus the SoC group was statistically and clinically significant in the following categories: FEV1 106 mL (P<0.001), 6-minute walk distance (6MWD) +39.31 m (P=0.002), St. George’s Respiratory Questionnaire (SGRQ) –7.05 points (P=0.004), hyperinflation (i.e., RV) –522 mL (P<0.001), modified Medical Research Council (mMRC) –0.8 points (P<0.001), and the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) score –1.2 points. The study authors concluded that the Zephyr® EBV provided clinically meaningful improvement in lung function, exercise capacity, and QoL at the 12-month mark. The most common complication was pneumothorax, which occurred in 26.6% of the EBV group subjects within the first 45 days.
Spiration® valve
The SVS was studied in the EMPROVE trial, which was a multicenter RCT in patients with heterogeneous emphysema (24). In this study, 172 subjects were randomized in a 2:1 ratio to either the SVS or SoC. At 12 months, the valve group had improvements in FEV1 (99 mL), SGRQ (–9.5 points), and 6MWD (+6.9 m). COPD exacerbations and pneumothorax were the most common adverse events. Within the first 6 months, the valve group experienced a 12.4% rate of serious pneumothorax, defined as those requiring tube thoracostomy drainage. As with the LIBERATE trial, the majority of these pneumothoraces occurred within the first 72 hours.
Survival
Survival after ELVR has been the subject of several recent studies (35-37). In a post hoc analysis of the STELVIO (Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation) trial, Klooster et al. suggested the potential for improved survival with EBV therapy based on improvements observed at 6 months in the BODE index, 6MWD, and inspiratory capacity to total lung capacity (IC/TLC) ratio, all of which have previously been shown to predict risk of death in patients with severe COPD (35,38).
In the most recent study, Gompelmann et al. demonstrated that lobar atelectasis following EBV insertion was associated with a significant 5-year survival advantage compared to those patients that did not develop atelectasis (65.3 vs. 43.9% 5-year survival rate; P=0.009) (37). Importantly, pneumothorax did not have a significant effect on survival (P=0.52). Pneumothorax is not necessarily indicative of procedural success; lobar atelectasis usually occurs without associated pneumothorax.
Ongoing studies
A number of studies are also ongoing. Several (Elevate study, Reaction study, Next Step study, and the STAGE trial) pertain to the non-EBV ELVR modalities that were not the focus of this review. The NCT03010449 trial is a single-arm study examining the combination of bronchoscopic autologous blood instillation in combination with EBVs. The NTR5007 trial is a single-arm study assessing the proactive treatment of CV in CV-positive patients before treatment with EBVs. The NCT03034421 trial is studying pneumothorax risk by randomizing patients to SoC or 48-hour bedrest after EBV insertion. The NCT03205826 study is examining the impact of Chartis® assessment performed under moderate sedation versus general anesthesia. The SOLVE trial is assessing the impact of pulmonary rehabilitation done either before or after EBV treatment. The NCT03518177 trial is designed to examine if there is any difference in effectiveness between home-based and supervised pulmonary rehabilitation in EBV candidates. Finally, the CELEB trial (ISRCTN19684749) is comparing LVRS and ELVR via EBVs head-to-head.
Guidelines
National and international guidelines (GOLD, NICE, etc.) have been developed to guide management (4,16). The GOLD guidelines provide the following information on interventional therapy in stable COPD:
- LVRS—improves survival in patients with severe emphysema that is upper-lobe predominant and who have a low exercise capacity (Evidence A);
- Bullectomy—associated with decreased dyspnea, improved pulmonary function, and exercise tolerance (Evidence C);
- Transplantation—improves QoL and functional capacity in select patients (Evidence C);
- ELVR—reduces end-expiratory lung volume and improves exercise tolerance, health status, and lung function (Evidence A for EBV, Evidence B for coils, & Evidence B for vapor ablation).
Conclusions
ELVR in general, and EBVs in particular, have become a viable option for patients with refractory respiratory symptoms despite optimal treatment of their emphysema. Numerous RCTs have demonstrated safety and clinical efficacy in the appropriate patient population. It may result in improved lung function, exercise tolerance, and QoL, without the morbidity and mortality of LVRS. Patient selection and optimal post-EBV insertion management remain areas of active research. Long-term outcomes beyond 12 months will need to be examined as data emerge.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “Interventional pulmonology and advanced bronchoscopy”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc-2019-ipab-15). The series “Interventional pulmonology and advanced bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. JSK served as the unpaid Guest Editor of the series and reports personal fees from Level Ex, personal fees from Medtronic, other from Pinnacle Biologics, personal fees from Biodesix, outside the submitted work. DKH served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest and reports personal fees from Olympus/Spiration, personal fees from PulmonX, during the conduct of the study; personal fees and other from Auris, personal fees from Ambu, personal fees, non-financial support and other from Body Vision, personal fees and other from Eolo, other from Eon, other from Gravitas, personal fees and other from Noah Medical, personal fees and other from LX-Medical, other from Med-Opsys, other from Monogram Orthopedics, personal fees and other from Preora, other from VIDA, other from Viomics, grants and personal fees from Boston Scientific, personal fees from Johnson and Johnson, personal fees from oncocyte, personal fees from veracyte, personal fees and other from Broncus, grants and personal fees from Gala, personal fees from Heritage Biologics, personal fees from IDbyDNA, personal fees from Level-Ex, personal fees from Medtronic, personal fees from Neurotronic, personal fees from olympus, personal fees from PulmonX, personal fees from Astra-Zeneca, personal fees from Biodesix, personal fees from Genetech, personal fees from Grifols, personal fees from Takeda, personal fees from CSL, personal fees from InhibRX, personal fees and other from Prothea-X, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442 [Crossref] [PubMed]
- Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448-57. [Crossref] [PubMed]
- Khakban A, Sin DD, FitzGerald JM, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am J Respir Crit Care Med 2017;195:287-91. [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;12:CD005305 [Crossref] [PubMed]
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev 2014;CD010844 [Crossref] [PubMed]
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;CD002309 [Crossref] [PubMed]
- Marchetti N, Criner GJ. Surgical approaches to treating emphysema: lung volume reduction surgery, bullectomy, and lung transplantation. Semin Respir Crit Care Med 2015;36:592-608. [Crossref] [PubMed]
- van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev 2016;10:CD001001 [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32. [Crossref] [PubMed]
- Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651-62. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Herth FJF. Bronchoscopic lung volume reduction in patients with severe emphysema. Semin Respir Crit Care Med 2018;39:685-92. [Crossref] [PubMed]
Guidelines GOLD 2020 . Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf- National Institute for Health and Care Excellence. Endobronchial valve insertion to reduce lung volume in emphysema. 2017. Available online: https://www.nice.org.uk/guidance/ipg600
- Zephyr® Endobronchial Valve System - P180002. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/zephyrr-endobronchial-valve-system-p180002
- Spiration® Valve System - P180007. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/spiration-valver-system-p180007
- Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med 2013;19:95-102. [Crossref] [PubMed]
- Hogg JC, Pare PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017;97:529-52. [Crossref] [PubMed]
- Welling JBA, Hartman JE, van Rikxoort EM, et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology 2018;23:306-10. [Crossref] [PubMed]
- Gülşen A. Bronchoscopic lung volume reduction: a 2018 review and update. Turk Thorac J 2018;19:141-9. [Crossref] [PubMed]
- Criner GJ, Delage A, Voelker K, et al. Improving lung function in severe heterogenous emphysema with the spiration valve system (EMPROVE). A multicenter, open-label randomized controlled clinical trial. Am J Respir Crit Care Med 2019;200:1354-62. [Crossref] [PubMed]
- Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198:1151-64. [Crossref] [PubMed]
- Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic lung volume reduction: an expert panel recommendation - update 2019. Respiration 2019;97:548-57. [Crossref] [PubMed]
- Li S, Wang G, Wang C, et al. The REACH trial: a randomized controlled trial assessing the safety and effectiveness of the Spiration® valve system in the treatment of severe emphysema. Respiration 2019;97:416-27. [Crossref] [PubMed]
- Kristiansen JF, Perch M, Iversen M, et al. Lobar quantification by ventilation/perfusion SPECT/CT in patients with severe emphysema undergoing lung volume reduction with endobronchial valves. Respiration 2019;98:230-8. [Crossref] [PubMed]
- Koster TD, van Rikxoort EM, Huebner RH, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration 2016;92:150-7. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014;19:524-30. [Crossref] [PubMed]
- Klooster K, Koster DT, Glösenkamp CR, et al. Functional measurement of collateral ventilation is of additional value to CT scan fissure analysis in endobronchial valve treatment for emphysema. Eur Respir J 2019;54:PA3405
- Wang Y, Lai TW, Xu F, et al. Efficacy and safety of bronchoscopic lung volume reduction therapy in patients with severe emphysema: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:78031-43. [Crossref] [PubMed]
- Franzen D, Straub G, Freitag L. Complications after bronchoscopic lung volume reduction. J Thorac Dis 2018;10:S2811-5. [Crossref] [PubMed]
- Valipour A, Slebos DJ, de Oliveira HG, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema--potential mechanisms, treatment algorithm, and case examples. Respiration 2014;87:513-21. [Crossref] [PubMed]
- Klooster K, Hartman JE, Ten Hacken NHT, et al. Improved predictors of survival after endobronchial valve treatment in patients with severe emphysema. Am J Respir Crit Care Med 2017;195:1272-4. [Crossref] [PubMed]
- Garner J, Kemp SV, Toma TP, et al. Survival after endobronchial valve placement for emphysema: a 10-year follow-up study. Am J Respir Crit Care Med 2016;194:519-21. [Crossref] [PubMed]
- Gompelmann D, Benjamin N, Bischoff E, et al. Survival after endoscopic valve therapy in patients with severe emphysema. Respiration 2019;97:145-52. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
Cite this article as: Kurman JS, Hogarth DK. Bronchoscopic lung volume reduction: who, what, where, when? Shanghai Chest 2021;5:29.


