The role of extrapleural pneumonectomy for malignant pleural mesothelioma: reviewing 20-years of experience
Introduction
Malignant pleural mesothelioma (MPM) is a very aggressive malignancy with a poor prognosis, requiring a complex treatment. Despite the fact that asbestos, the main etiologic factor, has been banned in most industrial countries for several decades, its global incidence is still increasing and MPM is expected to become a significant problem in third world countries in the next decades (1). In an adequately selected group of patients with resectable disease, a multimodality approach including neoadjuvant chemotherapy with platinum and anti-folate doublet, followed by surgical resection is part of the most recent guidelines (2-5). Due to the tumor’s anatomical restraint with proximity to the heart and the big vessels, the chest wall and the diaphragm, extensive resection is often not possible and microscopic tumor will eventually be left behind. The aim of a radical surgical treatment is therefore to achieve macroscopic complete resection (MCR) (4,6). MCR can be obtained by both extrapleural pneumonectomy (EPP) or extended pleurectomy and decortication (EPD). While EPP includes an en-bloc resection of the parietal pleura, the lung, the pericardium and the ipsilateral diaphragm, EPD forms a lung-sparing approach where only parietal and visceral pleura, the pericardium and the ipsilateral diaphragm are resected. An isolated pleurectomy and decortication (PD) can be evaluated in selected patients with no macroscopic or histological signs of tumor infiltration into the pericardium or diaphragm.
While EPP had already been established for treatment refractory tuberculosis in 1949 (7), EPP for MPM was only introduced in a series of 29 patients by Butchart et al. in 1976 (8). While in these patients, in-hospital mortality was as high as 31%, the safety of this procedure significantly improved over the following decades with short-term mortality ranging between 2.2% and 8.0% in recent reports (3,9-12). The technique of EPP has been described in previous articles and is largely consistent, including an extended lateral thoracotomy, extrapleural dissection of the tumor, as well as resection and reconstruction of the pericardium and the diaphragm (10). Increasing evidence from a number of retrospective cohort studies has demonstrated similar overall survival in EPP and EPD. Since EPP has been shown to be associated with a higher short-term mortality and morbidity, most centers have established EPD as a standard of care (3,12-14). The spared lung parenchyma and ideally preserved functional reserve after EPD results in improved quality of life and better tolerance towards further treatment (15). However, also in EPD the management of prolonged air leak and postoperative infections of the pleural cavity remain a challenge.
In this present article we aim to assess and discuss today’s role of EPP after induction chemotherapy followed by MCR, based on our own 20-year experience. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/shc-20-64).
Methods
Out of 523 patients with the diagnosis of MPM who underwent surgical resection within a multimodality concept at the Department of Thoracic Surgery of the University Hospital Zurich between January 1999 and December 2019, we identified 151 patients who underwent EPP. In all patients included in this study, the diagnosis of MPM had been histopathologically confirmed and a clinical T1–3 N0–2 M0 stage was present. A predicted postoperative forced expiratory volume in 1 second (FEV1) >1 L or >40% based on the preoperative spirometry was required for EPP. Perfusion was assessed routinely by ventilation/perfusion (V/Q) scan. In all cases, surgery was performed within a multimodality approach following three or four cycles of neoadjuvant chemotherapy with platinum-based agents and gemcitabine or pemetrexed. The combination of cisplatin and pemetrexed was used as a standard after improved survival rates had been shown in a randomized phase III trial in 2003 (16). In order to identify patients who will benefit from a multimodality approach, a multimodality prognostic score (MMPS) was established at our institution and has been shown to serve as an independent prognosticator for overall survival (OS) (17). In patients with an MMPS of 3 or 4 after neoadjuvant chemotherapy, the OS is not prolonged by subsequent radical surgery. The MMPS was therefore routinely assessed before surgical resection since its establishment in 2015. An approval of the local ethics committee was obtained for the retrospective analysis of the mesothelioma data base (StV 29-2009, EK-ZH 2012-0094).
Study endpoints
We aimed to assess perioperative mortality (in-hospital mortality, 30-day mortality, 90-day mortality), postoperative major morbidity and OS in our patient cohort. Major morbidity was defined as a composite outcome combining postoperative hemorrhage requiring surgery, a failure of the diaphragmatic or pericardial patch and/or diaphragmatic hernia, chylothorax, pleural empyema, bronchopleural fistula (BPF), acute respiratory distress syndrome (ARDS) and pulmonary embolism. For baseline characteristics of our cohort we collected data on gender, age, body mass index (BMI), preoperative lung function [FEV1, forced vital capacity exhaled (FVCex), diffusion capacity of carbon monoxide (DLCO)], smoking status, exposure to asbestos, MPM histology, the MMPS and neoadjuvant chemotherapy. The response to induction chemotherapy was evaluated by the modified response evaluation criteria in solid tumors (RECIST) and assessed using a dedicated reading workstation (Impax 5.2, AGFA, Bonn, Germany). The PET-CT scans before and after chemotherapy were linked at the identical anatomical position and tumor thickness was measured at three different levels in two distinct positions (18). MPM staging data are based on the TNM 8th edition [2016]. Mean tumor volume pre and post chemotherapy were assessed based on the staging positron emission computed tomography (PET-CT). During the reported 20 years, the diagnostic workup has changed and data on tumor volume, RECIST and the MMPS are therefore only available in more recent cases. The study population also included 52 patients who were included in the SAKK 17/04 randomized phase 2 trial (19). Of our cohort, 16 patients were included in the intervention arm and had received additional adjuvant high-dose hemithoracic radiotherapy.
Surgical technique
EPP was performed as a standardized procedure defined by an en-bloc resection of the parietal pleura and the lung via an extended lateral thoracotomy in the 6th intercostal space, as described previously (20). Using blunt force, the parietal pleura was dissected from the endothoracic fascia. In case of localized chest wall infiltration, the affected region of the chest wall was further excised. The parietal pleura was dissected from the thoracotomy towards the hilum. The pericardium or the diaphragm were resected if macroscopic or microscopic (confirmed by fresh frozen sections) infiltrations were present. Subsequently, the pulmonary ligament was divided and the hilum was approached. The pulmonary artery, as well as the superior and inferior pulmonary veins were divided intrapericardially and the main stem bronchus was closed using stapling devices. For reconstruction of the pericardium, an acellular biological patch was used and for reconstruction of the diaphragm, a Gore-Tex biomaterial patch was put in place and fixed to the chest wall. A systematic lymphadenectomy of the mediastinal lymph nodes was performed according to lung cancer surgery (right side: ATS 2R, 4R, 7, 10R; left side: ATS 5, 6, 7, 10L). If applicable, internal mammary artery nodes and intercostal nodes were resected as well.
Statistical analysis
Continuous variables are reported as mean ± standard deviation for normally distributed data and median with 95% confidence interval (CI) for non-normal distributions. Categorical variables are expressed as frequencies and percentages. Outcomes of the first and the second decade were compared using the unpaired t-test for continuous variables and using the Chi2-Pearson-test for categorical variables. Missing data (as well as loss to follow-up) was addressed by available case analysis. The 1-, 2-, and 3-year survival rates, as well as median OS were assessed by Kaplan-Meier curves. Statistical analysis was performed in IBM SPSS Statistics Version 24 (SPSS Inc, Chicago, IL).
Results
Baseline profiles
The baseline clinical profile of all 151 patients undergoing EPP for MPM is presented in Table 1. Of all patients, 87.4% were male and the mean age at the time of the operation was 60.0 years. Epitheloid MPM was the most commonly found histotype (63.6%), followed by biphasic (32.5%) and sarcomatoid (2.0%) histotype. After neoadjuvant chemotherapy, a partial remission was seen in 36.9% of all patients, whereas 37.9% showed a stable disease and 25.2% a progressive disease. At the time of re-staging by PET-CT after neoadjuvant chemotherapy, mean tumor volume was 294 cm3. The most common International Mesothelioma Interest Group (IMIG) stage was IB (50.3%), followed by stage IIIA (25.8%). Adjuvant radiotherapy was performed in 48.3% of all patients (n=73). Of these patients, 16 were included in the intervention arm of the SAKK 17/04 phase 2 trial and received adjuvant high-dose radiotherapy of the entire hemithorax and mediastinal nodal stations (19) and 52 patients were described previously in a propensity-matched comparison of EPP and EPD (12). Most patients were operated in the first decade between 1999 and 2009 (1st decade: 112 patients (74.2%); 2nd decade: 39 patients (25.8%). Patients operated during the second decade (2010–2019) were significantly older (62.2±8.3 vs. 59.3±7.0 years, P=0.036), had a significantly lower preoperative FEV1 (71.5%±15.2% vs. 79.6%±19.0%, P=0.019) and showed a higher IMIG stage (stage III in 51.3% vs. 34.8%).
Table 1
| Characteristic | Cohort: n=151 |
|---|---|
| Male gender | 132 (87.4%) |
| Right sided | 83 (55%) |
| Age at operation | 60.0±7.4 |
| BMI at operation | 25.0±3.3 |
| Asbestos exposure | |
| Possible | 31 (20.8%) |
| Confirmed | 89 (58.9%) |
| Smoking status | |
| Former | 62 (41.6%) |
| Current | 14 (9.4%) |
| Preoperative lung function | |
| FEV1 (%) | 77.3±18.3 |
| FVCex (%) | 81.5±19.4 |
| TLCO (%) | 71.6±17.9 |
| Neoadjuvant chemotherapy | |
| Platinum based/pemetrexed | 104 (68.9%) |
| Platinum based/gemcitabine | 46 (30.5%) |
| Other | 1 (0.7%) |
| Tumor volume pre chemotherapy (cm3)a | 320.9±312.9 |
| Tumor volume post chemotherapy (cm3)a | 294.6±315.0 |
| Histotype | |
| Epitheloid | 96 (63.6%) |
| Sarcomatoid | 3 (2.0%) |
| Biphasic | 49 (2.0%) |
| Undifferentiated | 3 (2.0%) |
| RECISTa | |
| Partial remission | 38 (36.9%) |
| Stable disease | 39 (37.9%) |
| Progressive disease | 26 (25.2%) |
| MMP-Scorea | |
| 0 | 21 (25.0%) |
| 1 | 42 (50.0%) |
| 2 | 15 (17.9%) |
| 3 | 5 (6.0%) |
| 4 | 1 (1.2%) |
| ypT stage (TNM 8th edition) | |
| 0 | 1 (0.7%) |
| 1 | 10 (6.6%) |
| 2 | 29 (19.2%) |
| 3 | 91 (60.3%) |
| 4 | 20 (13.2%) |
| ypN stage (TNM 8th edition)a | |
| 0 | 91 (60.3%) |
| 1 | 58 (38.4%) |
| IMIG stage | |
| IA | 11 (7.3%) |
| IB | 76 (50.3%) |
| II | 5 (3.3%) |
| IIIA | 39 (25.8%) |
| IIIB | 20 (13.2%) |
a, information not available for all patients. BMI, body mass index; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVCex, forced vital capacity exhaled, IMIG, International Mesothelioma Interest Group; RECIST, response evaluation criteria in solid tumors.
Surgical details
Details of the surgical procedure are shown in Table 2. In all but one patient, EPP included a resection and reconstruction of the diaphragm and the pericardium (99.3%). A systematic lymphadenectomy was performed in 91.4% of all cases. MCR could be achieved in 146 patients (96.7%).
Table 2
| Surgical characteristic: | Cohort: n=151 |
|---|---|
| Extrapleural pneumonectomy (EPP) | 151 (100%) |
| Resection of diaphragm | 150 (99.3%) |
| Resection of pericardium | 150 (99.3%) |
| Lymphadenectomy | 138 (91.4%) |
| Rib resection | 1 (0.7%) |
Outcome
Postoperative complications and the outcome after EPP are presented in Table 3. Seven patients (4.6%) died within 30 days after EPP. Death within 90 days after surgery occurred in 16 patients (10.6%).
Table 3
| Outcome characteristic: | Cohort: n=151 |
|---|---|
| Duration of hospitalization (days) | 17.3±11.3 |
| In-hospital mortality | 8 (5.3%) |
| 30-day mortality | 7 (4.6%) |
| 90-day mortality | 16 (10.6%) |
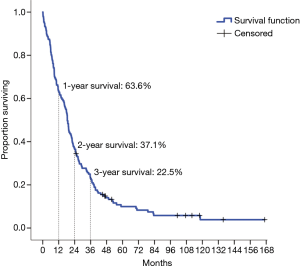
| Median survival, 95% CI (months) | 18.5 (1.1-90.5) |
| 1-year survival | 63.6% |
| 2-year survival | 37.1% |
| 3-year survival | 22.5% |
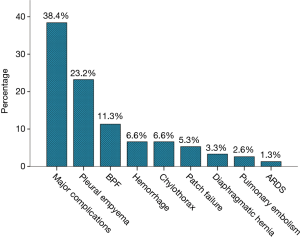
| Postoperative major morbidity | 58 (38.4%) |
| Reoperation | 53 (35.1%) |
| Pleural empyema | 35 (23.2%) |
| Bronchopleural fistula | 17 (11.3%) |
| Postoperative hemorrhage | 10 (6.6%) |
| Chylothorax | 10 (6.6%) |
| Diaphragmatic patch failure | 8 (5.3%) |
| Diaphragmatic hernia | 5 (3.3%) |
| Pulmonary embolism | 4 (2.6%) |
| ARDS | 2 (1.3%) |
ARDS, acute respiratory distress syndrome.
The 1-, 2- and 3-year OS were 63.6%, 37.1% and 22.5%, respectively and the median OS after EPP was 18.5 months (95% CI: 1.1–90.5 months) (Figure 1). In 39 patients who were operated after 2009, the median survival was increased to 22.0 months (95% CI: 0.8–112.2 months). However, no significant difference in the rate of major postoperative complications was found in patients operated during the first and during the second decade (36.6% vs. 43.6% respectively, P=0.71). The incidence of major postoperative morbidity as a composite outcome was 38.4% (Figure 2). Pleural empyema with or without BPF was the most common cause of major morbidity with an incidence of 23.2% and 11.3%, respectively, followed by chylothorax (6.6%) and postoperative bleeding requiring reoperation (6.6%). A diaphragmatic patch failure was encountered in 8 patients (5.3%), resulting in a subsequent diaphragmatic hernia in 5 patients (3.3%). In 35.1% of all patients, a reoperation was necessary. Tracheotomy after prolonged weaning was performed in 5 patients (3.3%). A transfusion of one or more blood products and postoperative atrial fibrillation were the most common minor complication, occurring in 45.7% and 33.8% of all patients, respectively.
Discussion
In this present single-center study we retrospectively analyzed the outcomes of 151 patients with MPM undergoing EPP at the University Hospital Zurich between January 1999 and December 2019. Our main findings show a 30-day short-term mortality of 4.6% and a median survival after surgery of 18.5 months. The perioperative mortality matches previous findings in the literature. In two meta-analyses by Taioli et al. and Cao et al., an increased perioperative mortality of 4.5 and 6.8%, respectively, was documented in patients undergoing EPP when compared to EPD. In our study cohort, major morbidity occurred in 38.4% and was most commonly caused by empyema. Empyema was treated according to our accelerated treatment concept with repeated radical surgical debridement of the pleural cavity, closure of BPF (if present), application of povidone-iodine soaked dressings, negative pressure wound therapy and systemic antibiotic therapy (21). The high rate of empyema and BPF has been previously described after EPP. It is hypothesized that a devascularized bronchial stump after parietal pleurectomy in combination with preceding chemotherapy may account for an increased rate of BPF in these patients (22,23). Atrial fibrillation was the most common minor complication, occurring in 33.8% of all patients, and has been previously reported in other cohorts (13,14,23,24). In relation to the high rate of perioperative major morbidity, the relatively low 30-day mortality demonstrates that complications after EPP can be successfully managed in experienced centers. In general, the center’s volume or case-load of patients plays an important role in morbidity and mortality after MPM surgery, as it has been demonstrated previously in an analysis of the STS general thoracic surgery database (25).
The median OS documented in previous studies assessing long-term outcomes after multimodality treatment with EPP varies between 12 and 22 months with a plateau commonly reached at about 18–22 months (26,27). In respect to the chosen surgical approach for MPM (EPP or EPD), the available data in the literature on OS are contradicting. While an analysis of the IASLC database showed an increased OS in patients with stage I undergoing EPP compared to EPD (40 vs. 23 months median survival) (28), other authors found no significant difference in overall survival (12,13) or even a significantly increased mortality in patients undergoing EPP (6,29). The only randomized controlled trial assessing the feasibility of a trimodal approach including induction chemotherapy, EPP, and postoperative hemithorax irradiation (MARS trial) had shown a limited OS after a trimodal treatment (14.4 vs. 19.5 months) (30). However, no valid conclusion could be drawn from the findings, since the trial lacked a sufficiently powered cohort and protocol compliance was inadequate (31,32).
The role of EPP has also been described for selected patients with pleural dissemination of other pleural malignancies, such as thymomas, low-grade sarcomas, and rare tumors such as hemangioendothelioma (33-37). For patients with pleural dissemination of thymoma, an excellent survival of up to 76% has been reported after EPP within a multidisciplinary approach (34,35,38,39).
In a study by Nakamura et al., EPP offered better OS and progression-free survival than an isolated resection of pleural dissemination in patients with sufficient cardiopulmonary reserve (38). For sarcoma and other rare thoracic malignancies, EPP is mostly only performed as a salvage procedure in otherwise healthy patients where other therapeutic options are exhausted (35,36,40).
In consideration of the baseline characteristics in our documented cohort with almost 40% of all patients having IMIG stage IIIA or IIIB and a mean preoperative tumor volume of 294.6 cm3 (Table 1), the present median survival of 18.5 months in our patient cohort is thus considered consistent with the previously documented OS. In the subgroup of patients who were operated after 2009, the median OS after EPP has further increased to 22.0 months. This increase is most probably multi-factorial and is assumed to represent the experience with the procedure, the postoperative management, the patient selection, as well as improvements in oncological and radio-oncological treatment after recurrence. This increase in OS is especially noteworthy since patients operated after 2010 were in general older, had a lower preoperative FEV1 and higher IMIG stages. However, the rates of postoperative complications did not differ between patients operated during the first and the second decade. A significant amount of the patients in our cohort underwent adjuvant radiotherapy, including 16 patients who received adjuvant hemithoracic radiation in the SAKK 17/04 randomized trial. The trial concluded that hemithoracic radiotherapy after EPP would rather provide an additional treatment burden without offering a survival benefit and this subgroup may further have affected the OS of the cohort (19). Since the interpretation of older follow-up CTs may be highly variable due to the long retrospective nature of the study in patients with EPP who underwent radical resections, progression-free survival was not assessed in this analysis.
For the preoperative selection of patients who may benefit from radical surgery in a multimodality concept, we nowadays only perform EPP or EPD in patients with an MMPS <3. In the past 20 years, EPP was performed in 6 patients with an MMPS >2. However, these patients were operated before the implementation of the MMPS in 2015 and the MMPS was retrospectively assessed during the process of its establishment.
In consideration of the increased perioperative morbidity after EPP for MPM and the high rates of local recurrence despite MCR, the role of EPP has been controversially discussed and lung-preserving solutions have come to the fore. This way, the preservation of the affected lung may help to tolerate possible further treatment (10). Due to the vast heterogeneity in biological behavior and due to the presumably small effect size between EPP and EPD in respect to OS, a large randomized controlled trial would be required to identify the ideal surgical approach from an oncological standpoint. Considering the limited OS in most patients despite radical treatment, it is to be discussed whether an optimization in OS or in quality of life should be considered as primary endpoint. At our institution, we analyzed QoL (preoperatively, 6 weeks and 4 months postoperatively) of patients diagnosed with MPM undergoing a multimodality therapy approach including either EPP or EPD (manuscript submitted). Our results show a trend in favor of (E)PD regarding certain items in the patient’s QoL. Nevertheless, EPP was not associated with an overall greater deterioration of QoL, consequently this procedure should still be part of the multimodality therapy approach.
This trend towards the lung-sparing approach raises the question in which cases an EPP would still be justified. In our center, EPD is the primarily chosen approach for patients with MPM scheduled for surgical resection with the intent of MCR. This is reflected by the fact that only one fourth (n=39, 25.8%) of all EPP patients in our cohort were operated in the past 10 years. However, the standard preoperative assessment of chest wall or parenchymal infiltration by computed tomography (CT) and magnetic resonance imaging (MRI) does not always provide sufficient soft tissue resolution to rule out the necessity of an extensive resection, namely by EPP (41). The decision on the surgical approach may thus only be made upon surgical exploration. We therefore recommend that all patients scheduled for MCR undergo a preoperative assessment by spirometry and V/Q-scan to predict postoperative FEV1 and determine whether the healthy lung offers sufficient quality and functional reserve to tolerate an EPP (27). Our present data confirm that despite recent trends towards EPD, EPP performed in an experienced center may still offer a reasonable approach in selected cases with large tumor burden and extensive involvement of the lung parenchyma, but good functional reserve of the non-affected lung (6,10,42,43). As a single-center study it needs to be highlighted, that external validity may be limited and the findings may not be generalizable. Moreover, the institution’s experience is crucial to prevent and possibly also manage postoperative complications such as ARDS or post-pneumonectomy empyema (14). This also includes the anesthesiologic experience to avoid high intraoperative FiO2 and barotrauma, both factors known to potentially trigger an ARDS after induction chemotherapy (6,14).
Conclusions
According to the current guidelines, a multimodality approach including neoadjuvant chemotherapy and MCR is recommended for selected patients. The past decade has shown a trend towards lung-sparing strategy (EPD) in most centers. In this current retrospective analysis of 151 patients who underwent EPP in the past 20 years, the main findings demonstrate that EPP is feasible in patients including advanced stage mesothelioma with high tumor burden and/or extensive infiltration into the lung parenchyma. However, patients need to be informed about the elevated morbidity and mortality with this approach and it should only be performed in experienced centers. In patients with locally advanced MPM, we recommend an individualized approach after thorough discussion at an interdisciplinary tumor board.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Strobe reporting checklist. Available at http://dx.doi.org/10.21037/shc-20-64
Data Sharing Statement: Available at http://dx.doi.org/10.21037/shc-20-64
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc-20-64). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Due to the aggressive character of the disease and the retrospective patient inclusion of up to 20 years, it was impossible to obtain an individual written consent from each patient. However, an approval of the local ethics committee was obtained for the retrospective analysis of our mesothelioma data base (StV 29-2009, EK-ZH 2012-0094).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 24A-24C.
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37. [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Kucukoner M, Ali Kaplan M, Inal A, et al. Clinical characteristics, treatment and survival outcomes in malignant pleural mesothelioma: an institutional experience in Turkey. J BUON 2014;19:164-70. [PubMed]
- Opitz I, Weder W. Pleural mesothelioma: is the surgeon still there? Ann Oncol 2018;29:1710-7. [Crossref] [PubMed]
- SAROT IA. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949;4:173-223. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. [Crossref] [PubMed]
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [Crossref] [PubMed]
- Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients. Interact Cardiovasc Thorac Surg 2017;24:740-6. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Schwartz RM, Lieberman-Cribbin W, Wolf A, et al. Systematic review of quality of life following pleurectomy decortication and extrapleural pneumonectomy for malignant pleural mesothelioma. BMC Cancer 2018;18:1188. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Opitz I, Friess M, Kestenholz P, et al. A New Prognostic Score Supporting Treatment Allocation for Multimodality Therapy for Malignant Pleural Mesothelioma: A Review of 12 Years' Experience. J Thorac Oncol 2015;10:1634-41. [Crossref] [PubMed]
- Frauenfelder T, Tutic M, Weder W, et al. Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 2011;38:162-8. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Werner RS, Caviezel C, Lauk O, et al. Extended pleurectomy and decortication with resection and reconstruction of pericardium and hemidiaphragm for malignant pleural mesothelioma. J Vis Surg 2020;6:20. [Crossref]
- Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: a binational long-term study. J Thorac Cardiovasc Surg 2008;136:179-85. [Crossref] [PubMed]
- Opitz I, Kestenholz P, Lardinois D, et al. Incidence and management of complications after neoadjuvant chemotherapy followed by extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2006;29:579-84. [Crossref] [PubMed]
- Lauk O, Hoda MA, de Perrot M, et al. Extrapleural pneumonectomy after induction chemotherapy: perioperative outcome in 251 mesothelioma patients from three high-volume institutions. Ann Thorac Surg 2014;98:1748-54. [Crossref] [PubMed]
- Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg 2014;97:1859-65. [Crossref] [PubMed]
- Bueno R, Opitz I, Taskforce IM. Surgery in Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1638-54. [Crossref] [PubMed]
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703. [Crossref] [PubMed]
- Opitz I, Weder W. A nuanced view of extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Transl Med 2017;5:237. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 6.e1-3.
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 4-5. [Crossref] [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery-mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83; discussion 83-4. [Crossref] [PubMed]
- Ruffini E, Filosso PL, Guerrera F, et al. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018;118:161-70. [Crossref] [PubMed]
- Wolf AS, Flores RM. Extrapleural pneumonectomy for pleural malignancies. Thorac Surg Clin 2014;24:471-5. [Crossref] [PubMed]
- Sugarbaker DJ TT, Swanson SJ, et al. The role of extrapleural pneumonectomy in the management of pleural cancers. J Clin Oncol 2009;27:7577. [Crossref]
- Takenaka M, Ichiki Y, Nabe Y, et al. Difficulty of treatment for pleural epithelioid hemangioendothelioma: a report of a case. Gen Thorac Cardiovasc Surg 2020;68:190-3. [Crossref] [PubMed]
- Nakamura S, Kawaguchi K, Fukui T, et al. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg 2019;67:524-9. [Crossref] [PubMed]
- Shiiya H, Hida Y, Kaga K, et al. Extrapleural pneumonectomy of recurrent thymoma with pleural dissemination. Respirol Case Rep 2018;6:e00308 [Crossref] [PubMed]
- Duranti L, Pardolesi A, Bertolaccini L, et al. Extra-pleural pneumonectomy. J Thorac Dis 2019;11:1022-30. [Crossref] [PubMed]
- Frauenfelder T, Kestenholz P, Hunziker R, et al. Use of computed tomography and positron emission tomography/computed tomography for staging of local extent in patients with malignant pleural mesothelioma. J Comput Assist Tomogr 2015;39:160-5. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Lausi PO, et al. Pleurectomy/decortication versus extrapleural pneumonectomy: a critical choice. J Thorac Dis 2018;10:S390-S394. [Crossref] [PubMed]
- Domen A, Berzenji L, Hendriks JMH, et al. Extrapleural pneumonectomy: still indicated? Transl Lung Cancer Res 2018;7:550-5. [Crossref] [PubMed]
Cite this article as: Werner RS, Lauk O, Opitz I. The role of extrapleural pneumonectomy for malignant pleural mesothelioma: reviewing 20-years of experience. Shanghai Chest 2020;4:40.