
Laparoscopic anti-reflux operation: fundoplication vs. Linx—techniques and outcomes
Introduction
Gastroesophageal reflux disease (GERD) is a globally prevalent condition affecting up to 28% of individuals in North America, 26% in Europe, and up to 8% in East Asia (1). The condition has significant negative effects on patient’s quality of life (QoL) and known long-term complications such as esophagitis, esophageal strictures, Barrett’s esophagus, and esophageal adenocarcinoma. First line treatment for GERD consists of medical therapy including a combination of diet and lifestyle modifications, as well as pharmacotherapy consisting of proton pump inhibitors (PPI) and H2 receptor antagonists with the ultimate goal of acid production reduction. Failure of medical management occurs in up to 40% of GERD patients and has been attributed to decreased tone of the lower esophageal sphincter (LES) and transient LES relaxation, but can also occur secondary to hiatal hernia or underlying esophageal dysmotility (2,3). The standard surgical management of GERD refractory to medical therapy is the Nissen fundoplication.
Nissen fundoplication technique
First described by Dr. Rudolf Nissen in 1955, the Nissen fundoplication aims at re-establishing LES pressure by wrapping the gastric fundus 360 degrees around the distal esophagus. Technical steps include dissecting the lower esophagus and creating a posterior esophageal window, intra-thoracic dissection of the esophagus to establish 2-3 cm of tension-free intra-abdominal esophagus segment, mobilization of the fat-pad usually situated over the gastroesophageal junction and of the posterior gastric fundus via division of the gastrophrenic ligament and short gastric vessels. A “floppy” 360° wrap (fundoplication) is fashioned using the gastric fundus around the esophagus. Repair of the diaphragmatic crura is commonly performed to ensure the integrity of the esophageal hiatus, and an esophageal bougie is frequently used to prevent over-tightening of the fundoplication (4). Since several steps are required to create a Nissen fundoplication, there is variability in how the procedure is performed among surgeons; and while results vary, they tend to be better in high-volume centers.
Nissen fundoplication outcomes and complications
The Nissen fundoplication achieves excellent long-term heartburn relief with 92.4% of patients reporting resolution in heartburn symptoms at 10 years, and 80% after 20 years (5-7). Still, up to 26% of Nissen fundoplication patients report postoperative persistence or recurrence of dysphagia, heartburn, and regurgitation. New symptoms following Nissen fundoplication include postprandial nausea, inability to belch or vomit, as well as bloating and dysphagia in up to 19.5% and 16.8% of patients, respectively (8,9). Surgical options for failed Nissen fundoplication include redo fundoplication or conversion to Roux-en-Y gastric bypass (RYGB) and have been shown to be both safe and effective, with RYGB more commonly used to revise failed fundoplication in obese patients (10,11). In a 2017 study by Schwameis et al., conversion from a Nissen to a Toupet fundoplication for control of post-fundoplication dysphagia and bloating relieved dysphagia in 84% and bloating in 100% of cases, with no significant surgical complications, supporting the efficacy of such revisional procedures (12). Ultimately, the Nissen fundoplication has been shown to offer long-term relief of GERD symptoms, with data supporting redo or revision fundoplication for symptom persistence, recurrence, or new postoperative gastroesophageal complaints.
Nissen fundoplication vs. PPI
Medical therapy of GERD has been evaluated against surgical intervention in several studies. In the LOTUS randomized trial, 92% of GERD patients on omeprazole PPI treatment were found to be in remission compared to 85% of patients having undergone laparoscopic Nissen fundoplication at five-year follow-up. However only 248 of 288 patients randomized to fundoplication underwent surgery. Additionally, residual symptom severity was worse in the PPI group, although complaints of bloating and flatulence were worse after fundoplication. Surgical treatment failure in this study was defined as need for any postoperative acid-suppressing drugs, reoperation for symptom control, or dysphagia requiring further treatment, possibly accounting for the lower remission rates post-fundoplication (13). The Nordic GERD group study determined that at 12 years, 45% of GERD patient on omeprazole with dose adjustment remained in remission compared to 53% of patient having undergone surgery (P=0.022). However, while the Nordic study found fundoplication to be superior to omeprazole in overall GERD symptom reduction, authors reported that the proportion of patients with post-surgical symptoms such as dysphagia, flatulence, impaired belching and inability to vomit did not decrease over the study’s follow-up period (14). Alternatively, Metha et al. demonstrated that of those patients who remained on PPI over a seven-year randomized control study, only 59% reported being satisfied with GERD symptom control vs. 80% of those who had undergone laparoscopic Nissen fundoplication (15).
Despite being the initial therapeutic standard of care, medical treatment of GERD through PPIs may not be optimal for all patients. Chronic PPI treatment has been suggested to contribute to increased risk of bone fractures and osteoporosis, renal failure, reduced clopidogrel activation leading to myocardial infarction, Clostridium difficile infection, anemia, hepatic encephalopathy and development of dementia (3,16). Likewise, certain patient may prefer to forego chronic, often life-long anti-reflux therapy and opt for surgery as a definitive intervention for GERD management. However, with new technologies emerging in the management of reflux symptoms, additional surgical options available to patients.
Linx device description and technique
The Linx Reflux Management System by Torax Medical is a LES magnetic augmentation device (MAS). First approved for use in humans by the FDA in 2012, the Linx device is implanted laparoscopically around the gastro-esophageal junction for treatment of GERD symptoms (17). The Linx functions on the premise that its expandable ring can provide sufficient resting pressure to the LES to prevent acid reflux symptoms, while allowing passage of food and liquid boluses with deglutination, as well as regurgitation of gastric contents when belching or vomiting. Devices implanted prior to 2015 are rated safe for MRI under 0.7 T, while those implanted in thereafter are rated for MRI up to 1.5T (17).
Currently, MSA device placement is approved for patients with GERD symptoms refractory to medical therapy, as demonstrated by abnormal pH testing. The studies considered for the FDA approval of the Linx device did not evaluate device placement in patients with hiatal hernias >3 cm, Barrett’s esophagus with LA classification C or D, esophageal motility disorders, prior anti-reflux procedures, morbidly obese patients with BMI >35, or in those under 21 years of age (17).
Placement of the Linx device is completed laparoscopically with several key steps: isolation and preservation of the hepatic branch of the anterior Vagus nerve, creation of a retro-esophageal window, posterior Vagus nerve identification, device sizing, and securing at the gastro-esophageal junction. While repair of hiatal defects is at the surgeon’s discretion during Linx placement, Tatum et al. found significantly lower rates of GERD recurrence and postoperative dysphagia in patients having undergone hiatus repair, with no recurrence of hernia or need for repeat hiatal hernia surgery in the crural repair group, supporting the completion of routine hiatal hernia repair with Linx placement (18).
Linx device outcomes
Postoperative outcomes of the Linx device have been favorable, with several short-term prospective single-arm studies reporting clinical improvements in GERD symptoms following MSA placement. Louie et al. reported that at one-year post-Linx device implantation, 87.4% of patients had completely discontinued PPI use, while in separate studies, 75.3% and 85% of patients reported a cessation of PPIs at 5-year follow-ups (19-21). In the same studies, postoperative esophageal acid normalization, defined as total percent time of pH <4 for <5.3% of the time, was achieved in 74% and 75% of patients at one- and five-year follow-up respectively (19,21). Subjectively, quality of life as reported via the Gastroesophageal Reflux Disease-Health Related Quality of Life questionnaire (GERD-HRQL) had significantly improved in 84% of participants in two independent studies reporting results at one- and five-year following surgery (19,20). Similarly, study participants’ post-MSA DeMeester scores were found to be normal in 72.4% of cases, with significant reduction in mean scores from 33.4 off PPIs to 12.0 at one-year follow-up (19). In contrast to post-Nissen fundoplication patients, multiple studies have demonstrated that over 90% of Linx patients retain the ability to belch and vomit if needed, whether at one- or five-year follow-up (19-21). Based on the limited available evidence with a majority of postoperative follow-up periods capped at five years, the current consensus is that MSA implantation is a safe and effective intervention for the management of symptomatic GERD, providing patients with significant objective and subjective relief from heartburn symptoms while preserving physiologic regurgitative functions.
Linx device complications
The most common postoperative complaint following Linx device placement is dysphagia, most prevalent in the immediate postoperative period with a reported 43% to 83% of patients experiencing difficult deglutination, and persistent dysphagia occurring in up to 19% of patients (22,23). This early postoperative dysphagia typically resolves within three months and is best managed symptomatically and through dietary adjustments; however, esophageal dilation and device removal may be necessary in some cases (3,24) . Factors found to be independent predictors of persistent post-MSA dysphagia were presence of preoperative dysphagia, having less than 80% peristaltic contraction on high-resolution impedance manometry, and normal hiatal anatomy (23). Esophageal dilation required for post-MSA dysphagia has been reported in 30.5% to 43.3% of patients, with post-dilation resolution rates of 67% to 76.9% (19,23). Alternatively, Ganz et al. demonstrated a non-significant increase in postoperative dysphagia following MSA placement, from 5% at one-year to 6% at five-year follow-up, while Bonavina et al. reported no evidence of dysphagia at patients’ last follow-ups over a six-year period from a preoperative dysphagia baseline of 8% (20,21). The above results illustrate the variable rates of post-MSA dysphagia, likely attributed to studies’ respective criteria for dysphagia, non-standardized surgical repair of the diaphragmatic crura, and subjective surgical MSA device sizing protocols.
Linx device removal
Reported Linx explantation rates vary from 1.1% to 6.7%, prompted by dysphagia, recurrent or persistent GERD symptoms, vomiting, chest pain, and device erosion. Most cited reasons leading to device removal include GERD symptom recurrence and dysphagia in up to 54% and 38% of cases, respectively (18-20). Notably, device erosion rates have been low per available literature, ranging from 0.1% to 1.2%. Approaches to Linx device removal include laparotomy, fully laparoscopic or endoscopic technique, as well as a two-step procedure of endoscopy followed by laparoscopy for esophageal erosion of MSA ring (18,25). Notable intraoperative findings at the time of device removal were new or expanding hiatal hernia with concomitant herniation of the MSA ring into the mediastinal space (70%), caudal displacement of the device below the GEJ (14%), and in some cases normal anatomy with adequate placement of the Linx device (18). For those patients found to have a hiatal hernia on device explantation for GERD symptoms, treatment options include MSA device replacement or fundoplication (typically Toupet) with optional crural repair as indicated (25). MSA replacement led to reflux symptom resolution in 75% of patients, while post-removal fundoplication patients were found to have a 33% resolution and 67% improvement of reflux symptoms. Alternatively, Tatum et al. have suggested refraining from additional interventions beyond device explantation in patients with postoperative dysphagia, especially in those with normal anatomy. At follow-up, 86% of post-MSA patients with dysphagia having undergone device removal alone were free of GERD symptoms and PPI use (18). The investigators theorized that the fibrotic encapsulation which forms around the Linx device continued to confer circumferential elastic strength to the GEJ even after ring explantation. Concomitant crura repair at the time of index MSA placement does not appear to negatively affect the rate of device explantation for dysphagia (26). In fact, certain high-volume centers now perform routine crura repair to prevent future recurrent or de novo hiatal hernias, and mitigate risks of possible device migration (18). Current data shows that of the small percentage of patients found to have MSA device erosion into the esophagus, all underwent successful device explantation without significant postoperative complications. However, given the short follow-up period of available studies, it is possible that higher rates of device erosion will be observed as time passes. Investigators suggest that patients who present with new or persistent symptoms of acid reflux or dysphagia post-Linx should be worked up thoroughly to rule out device malposition, hernia development, and the potentially dangerous event of ring erosion (18,26).
Linx device compared to PPI and fundoplication
As with any new surgical technology, the Linx MSA device is actively being compared to standard medical and surgical GERD therapies. The CALIBER study is a randomized control trial comparing the effectiveness of twice daily PPI vs. MSA placement for regurgitation symptoms in patients with moderate-to-severe regurgitation despite daily PPI use. In this study, investigator demonstrated that Linx was superior to twice daily PPI for control of GERD symptoms at twelve months follow-up, with complete elimination of regurgitation symptoms reported in 73% of MSA patients vs. 2% of PPI-only patients (P<0.001). Additionally, MSA patients experienced fewer bloating and rectal gas symptoms compared to those treated with PPI, and dysphagia decreased from 15% to 7% following MSA placement (P=0.0184) (27).
Several studies are emerging comparing fundoplication to MSA placement for control of GERD symptoms. In their retrospective propensity-matched cohort study comparing MSA to Nissen fundoplication at one-year follow-up, Warren et al. demonstrated similar outcomes in subjective reflux symptom control as quantified via the GERD-HRQL questionnaire, a non-significant difference in rate of persistent dysphagia requiring dilation, and noted a retained ability for belching and emesis in the MSA group. However, investigators did find that MSA patients had a significantly higher incidence of mild dysphagia, and had higher postoperative PPI use compared to Nissen (28). Similarly, Sheu et al. reported a higher incidence of patients with dysphagia requiring dilation post-MSA vs. Nissen fundoplication at short-term follow-up (29). Comparison of Linx placement against laparoscopic Toupet fundoplication with a minimum of one-year follow-up found no significant difference in reflux symptom control, PPI use, and bloating. While dysphagia rates were initially higher in MSA patients at three-months follow-up compared to Toupet patients, this difference dissipated by one-year follow-up (24). When scrutinizing the economic impact of MSA vs. Nissen, no significant difference was found between charges for Linx implantation compared to a laparoscopic Nissen fundoplication ($48,491 vs. $50,111), likely attributed to shorter operative time (66 vs. 82 min) and hospital length of stay (17 vs. 38 h) when MSA is performed. In this charge and outcomes study, GERD-HRQL scores for both interventions were again similar, and there was no significant difference in postoperative PPI use between the two groups (30). Table 1 highlights studies comparing Linx vs. fundoplication outcomes and side effects. Based on available evidence, MSA device placement for control of reflux symptoms is generally considered to be equivalent in safety and effectiveness when compared to the traditional laparoscopic Nissen or Toupet fundoplications. The use of Linx devices offers the advantages of retained belching and eructation ability, decreased postoperative bloating, and reversibility of procedure without major anatomical alterations. Still, higher initial postoperative rates of dysphagia are noted following MSA placement compared to traditional fundoplications, emphasizing the importance of patient selection and appropriate workup for Linx procedures.
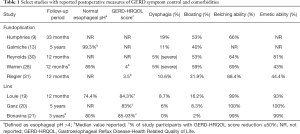
Full table
Special considerations for Linx
Given its efficacy and safety profile, the Linx device has been considered for treatment of non-traditional reflux symptoms. To date, bariatric surgery patients having undergone sleeve gastrectomy (SG) experiencing PPI-refractory GERD symptoms are offered a RYGB for combined reflux control and preserved weight-loss function. However, post-RYGB patients are reported to have acid reflux symptoms in 22% of cases, with up to 43.8% of patients using PPIs at one-year follow-up (32,33). Because of their altered gastric anatomy, RYGB, SG, and duodenal switch patients are not eligible for fundoplication, making the MSA device an attractive option for control of refractory GERD symptoms in the bariatric population. We have previously reported the successful use of Linx to treat persistent GERD after RYGB (34). Broderick et al. found that Linx placement for reflux symptoms in bariatric patients having undergone RYGB, SG, and DS led to either reduction or cessation of anti-reflux pharmacologic treatment in all patients, with significant reductions in GERD-HRQL scores and 100% patient satisfaction at the end of the 20 months follow-up period. Notably, two of 13 patients experienced persistent dysphagia requiring a single endoscopic dilation each with subsequent resolution of symptoms. The authors determined that, in carefully selected bariatric patients with refractory reflux symptoms, MSA device placement offers a safe and effective alternative to high-dose pharmacologic treatment or conversion to more complex surgery (35).
Current indications for Linx device placement are based on studies which excluded patients with hiatal hernia >3 cm (18). However, investigators are now reporting the use of Linx for patients with larger hiatal hernias. In their investigation of hernia recurrence following MSA placement, Rona et al. demonstrated a 4.3% recurrence rate at 18 months post-procedure in patients with moderate-sized hiatal hernias (3 to 7 cm), while Buckley et al. found 1.5% asymptomatic recurrence in their patients with large hernias, 78% of which had hernias >5 cm (26,36). In both studies, patients underwent complete hiatal hernia repair with stitch cruroplasty at time of MSA device placement, with 83% of patients’ primary hernia repair reinforced with non-permanent bioabsorbable mesh in the latter study. A total of 13% and 10% of patient experienced persistent dysphagia in each study, respectively. Rona et al. reported that two patients (3.8%) required device explantation, one for persistent dysphagia with subsequent conversion to Toupet fundoplication, and the other for refractory reflux symptoms with a recurrent 1 cm hernia leading to replacement of MSA device and repeat hiatal hernia repair. While further research is needed to assess the safety and efficacy of MSA implantation in patients with moderate-sized and large hiatal hernia, available data suggests that the Linx device may offer durable reflux symptoms relief in patient with moderate-sized hiatal hernia when concomitant hiatoplasty is performed (26,34,36).
Conclusions
To date, the gold standard for surgical treatment of GERD is the laparoscopic Nissen fundoplication. Based on emerging evidence, the Linx device appears to have comparable outcomes while offering a simple, reproducible, reversible, and anatomy-preserving alternative to the more extensive fundoplication. Dysphagia, frequently cited as a cumbersome early side effect, can be mitigated by diet modification in the post-operative period, with high rates of resolution following LES dilation when needed. With growing expertise, operative selection criteria are being expanded by high volume surgeons, in turn contributing to the expanding body of evidence supporting the appropriate use the MSA devices. Furthermore, both operative techniques and perioperative management are being honed by field experts, with crural repair and standardized postoperative diet being implemented with positive impact on patient outcomes. Still, future investigations are needed to assess the long-term outcomes of the Linx device and evaluate its safety and efficacy in a broader range of GERD patient such as those with large hiatal hernia, high-grade esophagitis, and bariatric surgery patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ghulam Abbas) for the series “Minimally Invasive Esophageal Surgery”, published in Shanghai Chest. This article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2020.02.01). The series “Minimally Invasive Esophageal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Hillman L, Yadlapati R, Thuluvath AJ, et al. A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease. Dis Esophagus 2017;30:1-15.
- Zadeh J, Andreoni A, Treitl D, et al. Spotlight on the LinxTM Reflux Management System for the treatment of gastroesophageal reflux disease: evidence and research. Med Devices (Auckl) 2018;11:291-300. [Crossref] [PubMed]
- Dallemagne B, Perretta S. Twenty years of laparoscopic fundoplication for GERD. World J Surg 2011;35:1428-35. [Crossref] [PubMed]
- Broeders JA, Rijnhart-De Jong HG, Draaisma WA, et al. Ten-year outcome of laparoscopic and conventional nissen fundoplication: Randomized clinical trial. Ann Surg 2009;250:698-706. [Crossref] [PubMed]
- Mardani J, Lundell L, Engström C. Total or posterior partial fundoplication in the treatment of GERD: Results of a randomized trial after 2 decades of follow-up. Ann Surg 2011;253:875-8. [Crossref] [PubMed]
- Frazzoni M, Piccoli M, Conigliaro R, et al. Laparoscopic fundoplication for gastroesophageal reflux disease. World J Gastroenterol 2014;20:14272-9. [Crossref] [PubMed]
- Catarci M, Gentileschi P, Papi C, et al. Evidence-Based Appraisal of Antireflux Fundoplication. Ann Surg 2004;239:325-37. [Crossref] [PubMed]
- Humphries LA, Hernandez JM, Clark W, et al. Causes of dissatisfaction after laparoscopic fundoplication: The impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc 2013;27:1537-45. [Crossref] [PubMed]
- Del Campo SEM, Mansfield SA, Suzo AJ, et al. Laparoscopic redo fundoplication improves disease-specific and global quality of life following failed laparoscopic or open fundoplication. Surg Endosc 2017;31:4649-55. [Crossref] [PubMed]
- Obeid NR, Altieri MS, Yang J, et al. Patterns of reoperation after failed fundoplication: an analysis of 9462 patients. Surg Endosc 2018;32:345-50. [Crossref] [PubMed]
- Schwameis K, Zehetner J, Rona K, et al. Post-Nissen Dysphagia and Bloating Syndrome: Outcomes After Conversion to Toupet Fundoplication. J Gastrointest Surg 2017;21:441-5. [Crossref] [PubMed]
- Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: The LOTUS randomized clinical trial. JAMA 2011;305:1969-77. [Crossref] [PubMed]
- Lundell L, Miettinen P, Myrvold HE, et al. Comparison of Outcomes Twelve Years After Antireflux Surgery or Omeprazole Maintenance Therapy for Reflux Esophagitis. Clin Gastroenterol Hepatol 2009;7:1292-8. [Crossref] [PubMed]
- Mehta S, Bennett J, Mahon D, et al. Prospective Trial of Laparoscopic Nissen Fundoplication Versus Proton Pump Inhibitor Therapy for Gastroesophageal Reflux Disease: Seven-Year Follow-up. J Gastrointest Surg 2006;10:1312-6. [Crossref] [PubMed]
- Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Vol. 153 Gastroenterology 2017;153:35-48. [Crossref] [PubMed]
- Telem DA, Wright AS, Shah PC, et al. SAGES technology and value assessment committee (TAVAC) safety and effectiveness analysis: LINX® reflux management system. Surg Endosc 2017;31:3811-26. [Crossref] [PubMed]
- Tatum JM, Alicuben E, Bildzukewicz N, et al. Minimal versus obligatory dissection of the diaphragmatic hiatus during magnetic sphincter augmentation surgery. Surg Endosc 2019;33:782-8. [Crossref] [PubMed]
- Louie BE, Smith CD, Smith CC, et al. Objective Evidence of Reflux Control After Magnetic Sphincter Augmentation. Ann Surg 2019;270:302-8. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Bonavina L, Saino G, Bona D, et al. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg 2013;217:577-85. [Crossref] [PubMed]
- Skubleny D, Switzer NJ, Dang J, et al. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc 2017;31:3078-84. [Crossref] [PubMed]
- Ayazi S, Zheng P, Zaidi AH, et al. Magnetic Sphincter Augmentation and Postoperative Dysphagia: Characterization, Clinical Risk Factors, and Management. J Gastrointest Surg 2020;24:39-49. [Crossref] [PubMed]
- Asti E, Bonitta G, Lovece A, et al. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: Observational cohort study with propensity score analysis. Medicine (Baltimore) 2016;95:e4366. [Crossref] [PubMed]
- Asti E, Siboni S, Lazzari V, et al. Removal of the Magnetic Sphincter Augmentation Device: Surgical Technique and Results of a Single-center Cohort Study. Ann Surg 2017;265:941-5. [Crossref] [PubMed]
- Rona KA, Tatum JM, Zehetner J, et al. Hiatal hernia recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate-term outcomes. Surg Endosc 2018;32:3374-9. [Crossref] [PubMed]
- Bell R, Lipham J, Louie B, et al. Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc 2019;89:14-22.e1. [Crossref] [PubMed]
- Warren HF, Brown LM, Mihura M, et al. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc 2018;32:405-12. [Crossref] [PubMed]
- Sheu EG, Nau P, Nath B, et al. A comparative trial of laparoscopic magnetic sphincter augmentation and Nissen fundoplication. Surg Endosc 2015;29:505-9. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Nieh A, et al. Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc 2016;30:3225-30. [Crossref] [PubMed]
- Riegler M, Schoppman SF, Bonavina L, et al. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc 2015;29:1123-9. [Crossref] [PubMed]
- Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2002;16:1027-31. [Crossref] [PubMed]
- Varban OA, Hawasli AA, Carlin AM, et al. Variation in utilization of acid-reducing medication at 1 year following bariatric surgery: Results from the Michigan Bariatric Surgery Collaborative. Surg Obes Relat Dis 2015;11:222-8. [Crossref] [PubMed]
- Muñoz-Largacha JA, Hess DT, Litle VR, et al. Lower Esophageal Magnetic Sphincter Augmentation for Persistent Reflux After Roux-en-Y Gastric Bypass. Obes Surg 2016;26:464-6. [Crossref] [PubMed]
- Broderick RC, Smith CD, Cheverie JN, et al. Magnetic sphincter augmentation: a viable rescue therapy for symptomatic reflux following bariatric surgery. Surg Endosc 2020;34:3211-5. [Crossref] [PubMed]
- Buckley FP, Bell RCW, Freeman K, et al. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc 2018;32:1762-8. [Crossref] [PubMed]
Cite this article as: Sterbling HM, Fernando HC. Laparoscopic anti-reflux operation: fundoplication vs. Linx—techniques and outcomes. Shanghai Chest 2021;5:9.


