Laparoscopic approaches to failed fundoplication for reflux
Introduction
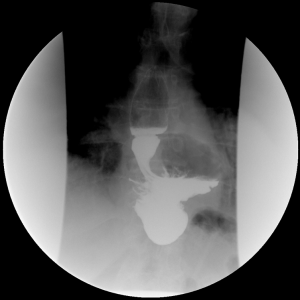
Laparoscopic primary fundoplication is now a well-established treatment for gastroesophageal reflux disease (GERD) (1), with failure rates ranging from 2% to 30% (2-7). The most common cause of failure of the primary fundoplication is transmediastinal migration of the wrap or recurrence of hiatal hernia (8) (Figure 1). Factors contributing to recurrence include failure to identify a short esophagus, inadequate crural closure, and improperly placed fundoplication (1,8). Most patients who present with symptoms of recurrent reflux after a fundoplication can be managed non-operatively with optimal medical therapy, endoscopic treatment, and lifestyle modification. However, 3% to 6% of patients with intractable GERD symptoms following primary fundoplication may benefit from re-operative surgical intervention (9). The surgical options for failed primary fundoplication for esophageal preservation include redo-fundoplication with or without an esophageal lengthening procedure, and Roux-en-Y near esophagojejunostomy (RNYNEJ). In select instances esophagectomy may be the only viable option.
Surgical approach to failed fundoplication
Surgical management of failed fundoplication is one of the most challenging foregut operations. These patients require extensive pre-operative assessment which includes review of prior operative records, a detailed history and physical examination, blood work including nutritional parameters, barium esophagram, endoscopy, and manometry. In select cases, a gastric emptying study and computed tomography (CT) may be warranted.
Patient preparation
Patients are placed on a clear liquid diet for 2 days prior to operation, and given a 1L polyethylene glycol electrolyte solution as a modified bowel prep. In the preoperative holding area, patients are given 5,000 units of heparin subcutaneously for venous thrombo-embolism (VTE) prophylaxis. Arterial monitoring is important in order to identify hemodynamic instability from possible capnothorax resulting from mediastinal dissection. Once the patient is positioned supine on the operating table, the arms are abducted to a 45-degree angle, and a foot board is placed to support steep reverse Trendelenburg. The surgeon is positioned to the right of the patient, with the assistant on the left.
Port placement
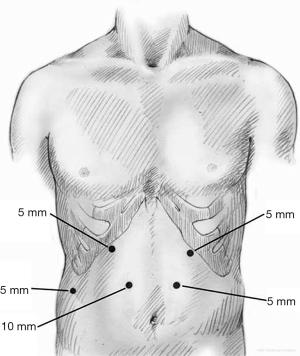
The abdomen is entered away from any previous incisions, usually in the right upper quadrant (RUQ), using a blunt port and cut down technique. Once the abdomen is insufflated to 15 mmHg, a 5 mm 30-degree camera is introduced and the abdominal cavity is inspected. The remaining ports are placed under direct visualization. Five ports are generally required to perform the procedure (Figure 2); one port at each subcostal midclavicular line, one paramedian port through each of the rectus muscles midway between xiphoid and umbilicus approximately 4 cm apart, and one subcostal right midaxillary line. All ports are 5 mm ports aside from the 10 mm right paramedian port. The patient is then placed in steep reverse Trendelenburg and a liver retractor (Lapro-Flex Triangular Retractor, Mediflex, Islandia, NY, USA) is introduced through the right midaxillary port to expose the right crus.
Key operative principles
The surgery begins with sharp dissection of adhesions between the liver and prior fundoplication. The caudate lobe is a key landmark which will aid in identification of the right crus. Once the right crus is identified, sharp dissection is continued to enter the mediastinum. This allows for safe identification of the esophagus and vagus nerves. It is imperative that the crural lining is preserved, as this aids in primary crural closure. If there was mesh used during the index operation, it is important to fully excise this in order to fully expose the hiatus. Once adequate dissection has been performed the hernia contents are then reduced. The right crus is completely mobilized from the caudate lobe if not done so previously. The left crus is dissected in a similar fashion, completely mobilizing free from the spleen. This will aid in tension free, primary crural closure.
If a large paraesophageal hernia (PEH) is present, the sac is identified and grasped within the hiatus at the 12-o’clock position and everted. A hemostatic energy device is then used to create a window between the hernia sac and the anterior peritoneal lining. This maneuver should expose an avascular areolar plane which will allow for dissection within the mediastinum along the posterior pericardium. Dissection within this plane should be carried up to the inferior pulmonary veins. The dissection is then carried out circumferentially to the level of the pleural lining bilaterally. The mediastinal dissection is then carried out posteriorly between the esophagus and the aorta, ensuring adequate mobilization between these structures as well. Once circumferential dissection of the esophagus is accomplished, mobilization of the right and left crura should be completed if not already achieved.
After complete mediastinal and crural mobilization, the previous fundoplication is taken down and normal anatomy is restored. This will not only allow for the assessment of the optimal type of repair but will often suggest the cause of failure of the index operation. Dissection is generally started along the left limb of the wrap, carefully sweeping away tissue centrally in order to avoid injuring the anterior vagus nerve. Removal of the fundoplication sutures should be approached from the undersurface of the wrap. Dissection of the right limb of the wrap is performed in the same manner. The short gastric vessels should also be divided completely if not already done. Adhesions to the stomach should be addressed, taking care to mobilize the posterior gastric attachments as well. Adequate mobilization of the stomach will allow it to lay in its normal anatomic position and will allow for the fundic tip to be easily lifted without resistance.
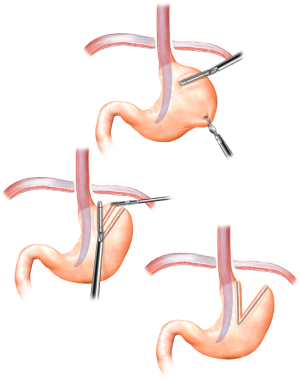
The next step is to determine adequate intra-abdominal esophageal length by dissection of the anterior gastroesophageal (GE) fat pad. The ideal tension free intra-abdominal esophageal length is 2.5 to 3 cm. It is critical identify the vagus nerves during the fat pad dissection to prevent injury. In cases where adequate tension free intra-abdominal esophageal cannot be obtained, a Collis gastroplasty is performed (Figure 3).
Once normal anatomy is restored and the GE junction fat pad is dissected, it is imperative to perform an on-table endoscopy to rule out any inadvertent esophageal or gastric perforation. If any injury is identified, it should be repaired at this time. If there is extensive damage to the esophagus, GE junction, or stomach, the surgical plan may need to be altered accordingly. Once endoscopic evaluation is complete, the type of surgical repair is then determined based upon objective preoperative work-up, patient characteristics, and intra-operative findings.
Crural repair
The preferred approach is a tension-free primary closure. Preservation of the crural integrity is essential for optimal outcome. Two to three interrupted 0 non-absorbable sutures are used to approximate the crura posterior to the esophagus (Figure 4). An anterior stich may also be required in certain instances. Very large defects pose a significant challenge to primary crural closure. Reducing the intra-abdominal pressure often aids in achieving a tension free repair. Another maneuver is inducing a left sided pneumothorax with close hemodynamic monitoring. Once the primary crural repair is achieved, a grasper is introduced through the hiatus to ensure an approximately 1 cm space around the esophagus. If tension free primary closure of the crura cannot be achieved or if the crural integrity is compromised, a biologic mesh can be used to close the defect. The mesh is secured to the diaphragm using 2-0 non-absorbable suture and a tacking device. Delayed erosion of non-absorbable mesh into the esophagus is a major concern, so its use should be avoided at all costs.
Redo-fundoplication ± Collis gastroplasty
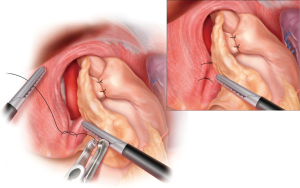
After crural repair, a redo fundoplication is performed in the following matter. A 52 to 56 Fr bougie is delivered trans-orally by the surgeon and positioned along the lesser curvature. If a Collis is required, it is completed at this time with the bougie in place (Figure 3). The fundus is passed, left to right, through the retroesophageal window, maintaining proper orientation using the line of the divided short gastric vessels. The “shoe-shine” maneuver is then performed confirming the proper orientation. The fundoplication is then secured using 2 simple interrupted 2-0 non-absorbable sutures. Each stitch should consist of a full thickness bite of stomach flanking a partial thickness bite of esophagus to prevent wrap herniation (Figure 5). The bougie is then removed after completion of the fundoplication followed by placement of nasogastric tube (NGT) under direct laparoscopic visualization. In cases where the patient has severe esophageal dysmotility, a partial wrap may be preferable.
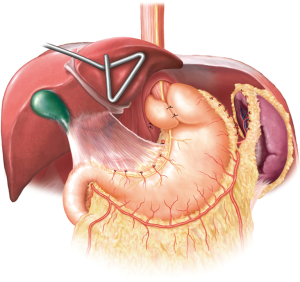
RNYNEJ
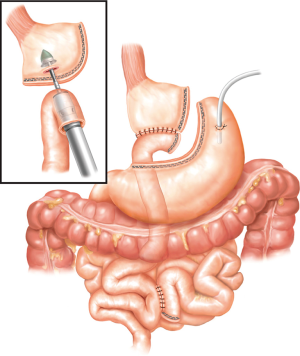
There is a certain subset of patients in whom redo-fundoplication may not be suitable, and these patients would benefit from a RNYNEJ. In these situations, once the normal anatomy is restored, a RNYNEJ is performed in the standard fashion with certain key elements to consider (10). Additional laparoscopic ports will be required to aid in the anastomoses. When constructing a RNYNEJ, it is important to have a small gastric pouch consisting of only cardia to minimize acid production. The Roux limb can vary between 60 to 100 cm based on body mass index. It is typically positioned retrogastric and retrocolic. The proximal anastomosis is performed using a preloaded anvil on an orogastric tube (Orvil, Medtronic, Minneapolis, MN, USA) and an EEA stapler (Medtronic, Minneapolis, MN, USA) (Figure 6) while the distal anastomosis is constructed with a linear GIA stapler (Medtronic, Minneapolis, MN, USA). All potential defects are closed. Typically, a JP drain is placed behind the proximal anastomosis, and in select cases a decompressive gastrostomy tube is considered. If the cardia is not suitable for anastomosis, then an esophagojejunostomy should be performed (Figure 7).

Other reconstructive options
In cases where the esophagus is severely diseased, damaged, or devascularized, esophagectomy may be the only viable option. Our preference is to complete a minimally invasive Ivor Lewis esophagectomy, which we described previously in detail (12) (Figure 8). A gastropexy may be of benefit in limited circumstances in situations where the esophagus is preserved, however, the fundus is not suitable for a fundoplication. If constructing a gastropexy, we prefer to tack the stomach to the diaphragm rather than use a gastrostomy tube as a form of pexy. We construct the gastropexy by suturing the stomach along the divided short gastric line to the diaphragm using a series of horizontal mattress sutures. The stomach is oriented laterally toward the spleen in order to recreate its natural position.
Comments
Re-operative intervention for failed fundoplication is very complex and poses a significant challenge to the esophageal surgeon. Re-operative anti-reflux surgery is associated with increased morbidity (13). A subset of patients will require further operations (14). The success rate for re-operative anti-reflux surgery decreases with each subsequent reoperation. Symptomatic control after first time fundoplication is achieved in 90% of patients (15) however outcomes drop to 60–80% after one or more re-operative anti-reflux surgery (16). Some patients that present with recurrent symptoms after a failed fundoplication can be treated non-operatively with a combination of diet and lifestyle modification as well as optimal medical management. However, patients with intractable symptoms and defined anatomical recurrence may benefit from re-operative intervention.
Re-operative intervention for failed fundoplication can be performed through a minimally invasive approach with good results (14,17-20). In a recent study performed at the University of Pittsburgh, re-operative results for failed fundoplication were analyzed. The most common pattern of failure of the index operation was transmediastinal migration in 177 patients (64%). Operative interventions for failed fundoplication included Nissen fundoplication in 200 patients (73%), Collis gastroplasty in 119 patients (43%), and partial fundoplication in 41 patients (15%). There was no perioperative mortality, and the 2-year estimated probability of freedom from failure was 93%. The health-related quality of life (HRQOL) scores were also noted to be excellent to satisfactory in 85.5% of patients (14).
Experience with RNYNEJ is also well documented by another study performed at the University of Pittsburgh. One hundred five patients with BMI greater than 25 underwent RNYNEJ for failed anti-reflux operations. Most were obese [BMI >30; 82 patients (78%)], and esophageal dysmotility was demonstrated in more than one-third of patients. Forty-eight (46%) patients had multiple anti-reflux operations before RNYNEJ, and 27 patients had undergone a previous Collis gastroplasty. There was no perioperative mortality. During follow-up (mean, 23.39 months), median BMI decreased from 35.0 to 27.6 (P<0.0001), and the mean dysphagia score decreased from 2.9 to 1.5 (P<0.0001). The median GERD HRQOL score, assessed in a subset of patients, was classified as excellent (21). This study not only showed that RNYNEJ is a viable and effective alternative to fundoplication in the correct setting, but also showed benefit for patients with obesity, and esophageal dysmotility. Other studies have also demonstrated the effectiveness of Roux-en-Y reconstruction after failed fundoplication in regards to GERD symptom control (10,22-24). Another study by Juhasz et al. demonstrated that Roux-en-Y reconstruction produced significantly better results for patients with a short esophagus compared to a redo Collis gastroplasty (19).
Another option for carefully selected patients is the use of a gastropexy. Ideal patients for this procedure are those with minimal reflux history as this procedure may put the patient at risk for reflux. Instances where this procedure may be appropriate are for patients with multiple and significant comorbid conditions, elderly patients, patients with significant esophageal motility disorders, or when the viability of the fundus is in doubt. In these instances, a gastropexy may offer a better functional outcome however further investigation of outcomes after gastropexy are needed before any definitive conclusion can be made.
Esophageal preservation in the treatment of failed fundoplication is recommended. Unfortunately for patients with severe esophageal dysmotility, multiple prior operations, strictures, obstructive symptoms, and/or high-grade dysplasia/cancer (25) this may not always be feasible. In such cases esophagectomy has been performed with good results (26). However, esophagectomy is associated with higher morbidity (25,27). Furthermore, esophagectomy for failed fundoplications are associated with a higher use of non-gastric conduit (26). As such when esophagectomy is being considered for operative intervention, alternative conduits should be evaluated preoperatively. Although a viable option, esophagectomy for failed fundoplication remains challenging and carries a high mortality rate. It should only be performed by experienced esophageal surgeons at high-volume centers.
Conclusions
In conclusion the surgical management for failed fundoplication is highly complex, and requires extensive pre-operative work-up. Despite this, re-operative intervention for failed fundoplication can usually be accomplished with minimally invasive techniques. Surgical options include redo-fundoplication, RNYNEJ, gastropexy, or esophagectomy. Optimal surgical option is dependent on pre-operative work-up, intra-operative findings, and patient characteristics. Good to excellent outcomes can be achieved for re-operative anti-reflux surgery when performed by experienced esophageal surgeons at high volume centers.
Surgical pearls
- Details such as esophageal mobilization, vagal preservation, division of the short gastric vessels, creation of a fundoplication, and crural repair must be obtained on review of operative records. These details will allow for a better understanding of the patient’s anatomy and will provide further insight into the technical causes surrounding the failure of the primary fundoplication.
- If dense adhesions are encountered, a key anatomical landmark to identify is the caudate lobe of the liver, which will aid in identifying the right crus.
- During dissection of the right and left crura, it is imperative to preserve the crural integrity, as this will optimize the success of primary repair.
- Early identification of the vagus nerves is key to prevent injury during dissection.
- The hernia sac may be densely adherent to the pleural lining and violation of the pleural space may result in capnothorax. Although usually asymptomatic, tension physiology may occur. This can easily be resolved with timely chest tube or pigtail catheter placement.
- Patients that may benefit from a RNYNEJ are obese patients with comorbidities such as diabetes, hyperlipidemia, and sleep apnea. They may also have some component of esophageal dysmotility.
- Patients that may benefit from esophagectomy include: severe esophageal dysmotility, multiple prior redo-foregut surgeries, strictures, symptoms of obstruction, esophageal ischemia, or dysplasia.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ghulam Abbas) for the series “Minimally Invasive Esophageal Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2020.02.03). The series “Minimally Invasive Esophageal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ohnmacht GA, Deschamps C, Cassivi SD, et al. Failed antireflux surgery: results after reoperation. Ann Thorac Surg 2006;81:2050-3; discussion 2053-4. [Crossref] [PubMed]
- Little AG, Ferguson MK, Skinner DB. Reoperation for failed antireflux operations. J Thorac Cardiovasc Surg 1986;91:511-7. [Crossref] [PubMed]
- DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease. Evaluation of primary repair in 100 consecutive patients. Ann Surg 1986;204:9-20. [Crossref] [PubMed]
- Hiebert CA, O'Mara CS. The Belsey operation for hiatal hernia: a twenty year experience. Am J Surg 1979;137:532-5. [Crossref] [PubMed]
- Peters JH, DeMeester TR. Indications, benefits and outcome of laparoscopic Nissen fundoplication. Dig Dis 1996;14:169-79. [Crossref] [PubMed]
- Hunter JG, Trus TL, Branum GD, et al. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg 1996;223:673-85; discussion 685-7. [Crossref] [PubMed]
- Smith CD, McClusky DA, Rajad MA, et al. When fundoplication fails: redo? Ann Surg 2005;241:861-9; discussion 869-71. [Crossref] [PubMed]
- Awais O, Luketich JD, Schuchert MJ, et al. Reoperative antireflux surgery for failed fundoplication: an analysis of outcomes in 275 patients. Ann Thorac Surg 2011;92:1083-9; discussion 1089-90. [Crossref] [PubMed]
- Morse C, Pennathur A, Luketich JD. Laparoscopic techniques in reoperation for failed antireflux repairs. In: Patterson GA, Pearson FG, Cooper JD, et al. editors. Pearson’s textbook of thoracic and esophageal surgery. Philadelphia: Churchill Livingstone, 2008:367-75.
- Awais O, Luketich JD, Tam J, et al. Roux-en-Y near esophagojejunostomy for intractable gastroesophageal reflux after antireflux surgery. Ann Thorac Surg 2008;85:1954-9; discussion 1959-61.
- Steven A. Tamesis, Tadeusz D. Witek, Omar Awais. Roux-en-Y near esophagojejunostomy. Asvide 2020;7:075. Available online: http://www.asvide.com/watch/33114
- Pennathur A, Awais O, Luketich JD. Technique of minimally invasive ivor lewis esophagectomy. Ann Thorac Surg 2010;89:S2159-62. [Crossref] [PubMed]
- Little AG, Ferguson MK, Skinner DB. Reoperation for failed antireflux operations. J Thorac Cardiovasc Surg 1986;91:511-7. [Crossref] [PubMed]
- Awais O, Luketich JD, Schuchert MJ, et al. Reoperative antireflux surgery for failed fundoplication: an analysis of outcomes in 275 patients. Ann Thorac Surg 2011;92:1083-9; discussion 1089-90. [Crossref] [PubMed]
- Pessaux P, Arnaud JP, Delattre JF, et al. Laparoscopic antireflux surgery: five-year results and beyond in 1340 patients. Arch Surg 2005;140:946-51. [Crossref] [PubMed]
- Furnée EJ, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009;13:1539-49. [Crossref] [PubMed]
- Kao AM, Otero J, Schlosser KA, et al. One more time: redo paraesophageal hernia repair results in safe, durable outcomes compared with primary repairs. Am Surg 2018;84:1138-45. [PubMed]
- Wennergren J, Levy S, Bower C, et al. Revisional paraesophageal hernia repair outcomes compare favorably to initial operations. Surg Endosc 2016;30:3854-60. [Crossref] [PubMed]
- Juhasz A, Sundaram A, Hoshino M, et al. Outcomes of surgical management of symptomatic large recurrent hiatus hernia. Surg Endosc 2012;26:1501-8. [Crossref] [PubMed]
- van Beek DB, Auyang ED, Soper NJ. A comprehensive review of laparoscopic redo fundoplication. Surg Endosc 2011;25:706-12. [Crossref] [PubMed]
- Awais O, Luketich JD, Reddy N, et al. Roux-en-Y near esophagojejunostomy for failed antireflux operations: outcomes in more than 100 patients. Ann Thorac Surg 2014;98:1905-11; discussion 1911-3.
- Kellogg TA, Andrade R, Maddaus M, et al. Anatomic findings and outcomes after antireflux procedures in morbidly obese patients undergoing laparoscopic conversion to Roux-en-Y gastric bypass. Surg Obes Relat Dis 2007;3:52-7; discussion 58-9. [Crossref] [PubMed]
- Washer GF, Gear MW, Dowling BL, et al. Randomized prospective trial of Roux-en-Y duodenal diversion versus fundoplication for severe reflux oesophagitis. Br J Surg 1984;71:181-4. [Crossref] [PubMed]
- Salo JA, Lempinen M, Kivilaakso E. Partial gastrectomy with Roux-en-Y reconstruction in the treatment of persistent or recurrent oesophagitis after Nissen fundoplication. Br J Surg 1985;72:623-5. [Crossref] [PubMed]
- Madenci AL, Reames BN, Chang AC, et al. Factors associated with rapid progression to esophagectomy for benign disease. J Am Coll Surg 2013;217:889-95. [Crossref] [PubMed]
- Chang AC, Lee JS, Sawicki KT, et al. Outcomes after esophagectomy in patients with prior antireflux or hiatal hernia surgery. Ann Thorac Surg 2010;89:1015-21; discussion 1022-3. [Crossref] [PubMed]
- Shen KR, Harrison-Phipps KM, Cassivi SD, et al. Esophagectomy after anti-reflux surgery. J Thorac Cardiovasc Surg 2010;139:969-75. [Crossref] [PubMed]
Cite this article as: Tamesis SA, Witek TD, Luketich JD, Awais O. Laparoscopic approaches to failed fundoplication for reflux. Shanghai Chest 2020;4:33.