
A review of the modern, multidisciplinary approach to the management of massive hemoptysis
Overview
Massive hemoptysis is the life-threatening expectoration of large volumes of blood as a result of tumor, infection, or other cause. Historically, it has been managed with emergent thoracotomy, however, advancements in interventional pulmonology and interventional radiology have transformed the treatment algorithm for massive hemoptysis. Endobronchial and endovascular therapies are now often utilized as temporizing measures to control hemorrhage and bridge to more definitive therapies such as chemotherapy, antibiotics, or surgery. Management can be complex and is best handled by a well-trained, multidisciplinary team. In the following paragraphs, we review a detailed, modern approach to the management of massive hemoptysis.
Definition
Hemoptysis can range from blood-streaked sputum to substantial pulmonary hemorrhage, but massive hemoptysis accounts for only 5% to 15% of events (1,2). While rare, massive hemoptysis is deadly. Mortality rates can reach 80% if not appropriately managed (3). There is no consensus on the definition of “massive” hemoptysis. Proposed definitions include volumes ranging from 100 to 1,000 mL of blood over a 24-hour period. Others are based on rate of bleeding per hour over a 24-hour period (2). It can be difficult, however, for patients to accurately quantify hemoptysis, and under-reporting and exaggeration of blood loss are common. This has led to definitions based on “magnitude of effect” or “clinical consequence,” whereby “massive” means the volume of blood that results in transfusion, hypoxemia, intubation, shock, or even death (1).
Anatomy
The lungs are supplied by both pulmonary and bronchial arteries. The pulmonary arterial system is a low-pressure system that accounts for only 5% of cases of massive hemoptysis (2). Blood is carried from the right ventricle to the pulmonary capillary bed where gas exchange occurs. Blood is then returned to the left ventricle via the pulmonary veins. Mean pressures in this circulation are 15–20 mmHg systolic and 5–10 mmHg diastolic (1).
The majority (90%) of cases of massive hemoptysis can be localized to the bronchial circulation, a high-pressure system arising directly or indirectly from the aorta. It receives 1% to 2% of cardiac output and provides blood flow to the tracheobronchial tree. It also supplies the hilar lymph nodes, visceral pleura, pulmonary arteries and veins, vagus nerve, and esophagus (1). The bronchial arteries arise from the descending aorta between the third and eighth thoracic vertebral bodies, most commonly between T5 and T6. Nearly 40% of patients have a single right bronchial artery and two left bronchial arteries arising from T5/T6, but anatomical variations are common (2). The final 5% of cases of massive hemoptysis arise from the aorta itself or from the non-bronchial systemic circulation, which includes the intercostal, coronary, and thoracic arteries (1).
Etiology
The differential diagnosis for the etiology of massive hemoptysis is broad and varies by clinical setting and geography (Table 1). In the 1960s, Mycobacterium tuberculosis, bronchiectasis, and lung abscesses accounted for 90% of massive hemoptysis (1). Today, the most common causes include bronchiectasis, tuberculosis, mycetomas, necrotizing pneumonia, and lung malignancy (2). About 20% of lung cancer patients experience hemoptysis, but only 3% of patients present with massive hemoptysis (1). In cystic fibrosis, the annual incidence of massive hemoptysis is 1%, though prevalence increases in later stages of the disease (4). A recent retrospective cohort study looked at hemoptysis in adults over a 5-year period using the French nationwide hospital administrative database. The authors found that 18% of cases of hemoptysis are cryptogenic, despite advancements in diagnostics and therapeutics (5).
Table 1
| Etiologies of massive hemoptysis |
| Malignancy |
| Bronchogenic carcinoma |
| Endobronchial tumor |
| Pulmonary metastases |
| Sarcoma |
| Infectious |
| Lung abscess or necrotizing pneumonia |
| Endocarditis with septic emboli |
| Mycetoma |
| Mycobacteria |
| Parasitic disease |
| Cardiac/pulmonary vascular |
| Pulmonary artery aneurysm |
| Pulmonary hypertension |
| Pulmonary embolism/infarct |
| Congenital heart disease |
| Mitral stenosis |
| Left heart failure |
| Pulmonary veno-occlusive disease |
| Dieulafoy’s lesion |
| Arteriovenous malformation |
| Bronchovascular fistula |
| Pulmonary |
| Bronchiectasis |
| Chronic bronchitis |
| Diffuse alveolar hemorrhage |
| Foreign body aspiration |
| Broncholithiasis |
| Lung transplantation |
| Idiopathic pulmonary hemosiderosis |
| Lymphangioleiomyomatosis |
| Vasculitis/collagen vascular disease |
| Granulomatosis with polyangiitis |
| Systemic lupus erythematosus |
| Behcet’s disease |
| Goodpasture’s syndrome |
| Other vasculitis or collagen vascular disease |
| Hematologic |
| Coagulopathy |
| Platelet disorder |
| Drugs & toxins |
| Crack cocaine |
| Penicillamine |
| Solvents |
| Nitrofurantoin |
| Bevacizumab |
| Iatrogenic/trauma |
| Catheter-induced pulmonary artery rupture |
| Blunt or penetrating chest injury |
| Secondary to biopsy, bronchoscopic procedure |
| Airway stent |
| Other |
| Cryptogenic |
| Endometriosis |
| Tuberous sclerosis |
Diagnosis & management (Table 2)
Table 2
| Overview of the management of massive hemoptysis |
| Initial approach & stabilization |
| Diagnosis & localization |
| Treatment |
| Endobronchial therapies |
| Thermal ablative techniques |
| Argon plasma coagulation |
| Electrocautery |
| Laser therapy |
| Bronchial-blocking balloon catheters |
| Silicone spigots |
| Endobronchial valves |
| Endobronchial sealing |
| Endovascular therapies |
| Bronchial artery embolization |
| Tranexamic acid |
| Surgery |
Initial approach & stabilization
Massive hemoptysis is a medical emergency that requires a multidisciplinary approach to management. As is the first step in any emergency, the patient’s airway and circulation must be stabilized. Large-bore peripheral IVs or large-bore central access need to be secured. Complete blood count, blood type, cross-match, and coagulation profile should be obtained to guide resuscitation. The patient should be positioned with the bleeding side down, if this is known, to allow gravity to isolate the bleeding lung.
An airway needs to be established as efficiently as possible to prevent asphyxiation. A large-lumen endotracheal tube (ETT) is the most accessible option in most settings. An appropriate ETT will have an internal diameter of 8.5 to 9 mm to facilitate use of fiberoptic bronchoscopy (FOB). FOB can guide the ETT through the vocal cords and into the non-bleeding mainstem bronchus. Disadvantages of single lung intubation include inability to intervene upon the bleeding lung and right upper lobe collapse with intubation of the right mainstem bronchus (2).
Another option for airway stabilization is a double-lumen ETT (DLETT), which consists of a long lumen and a short lumen bound together. The longer lumen is advanced into a mainstem bronchus while the shorter lumen remains in the trachea to ventilate the other lung (3). FOB confirms proper placement, as positioning can be difficult and time-consuming. A pediatric bronchoscope can be advanced through one of the lumens, but it is not large enough to remove blood rapidly and tends to clog. For these reasons, the DLETT is not recommended as a first-line approach in cases of massive hemoptysis.
Rigid bronchoscopy offers the advantage of simultaneous visualization and ventilation. It also is the most efficient approach to airway stabilization. Blood and clots can be rapidly evacuated, and instruments can be exchanged quickly. The rigid scope can isolate a bleeding lung or core out tumor. FOB can be used concomitantly to localize the bleed without compromising ventilation or facilitate use of the endoscopic therapies described below (6). Unfortunately, not all bronchoscopists are trained in rigid bronchoscopy, and rigid scopes are not available in all bronchoscopy suites.
Once the patient’s airway and circulation are stabilized, additional history can be obtained, including the patient’s procedural history and current medication list. Coagulopathies should be corrected. The interventional radiology, interventional pulmonology, and thoracic surgery teams should be kept apprised of the patient’s status so that multidisciplinary decisions can be made.
Diagnosis & localization
While chest radiography can generally depict the site of bleeding in 45% to 65% of cases of massive hemoptysis, FOB or computed tomography (CT) are more likely to localize a bleed (3). Revel et al. reviewed chest X-rays and CT scans from 80 patients with “large” or “massive” hemoptysis. The site of bleed was identifiable on 46% of plain films compared to 70% of CT scans. FOB was performed in 73 of the 80 patients and identified the site of bleed in 73% of patients. When assessing diagnostic yield, however, the authors found that the cause of bleeding was identified on 35% of chest X-rays, 77% of CT scans, and only 8% of bronchoscopies, suggesting that CT is superior to bronchoscopy as a diagnostic tool (7). The authors did point out that bronchiectasis, post-tuberculous lesions, and aspergillomas accounted for more than half of the causes of bleeding in the study.
In another study looking at 40 cases of hemoptysis with normal bronchoscopy, abnormalities were seen on subsequent CT scan in 50% of cases (8). Similarly, in a prospective study comparing CT to bronchoscopy in 91 cases of hemoptysis, CT identified the 27 tumors seen on bronchoscopy plus seven additional lesions. CT also found 14 cases of bronchiectasis that were not detected on FOB (9). Unfortunately, CT requires the patient to be stable enough to travel to the scanner. In unstable patients or in patients with bilateral lung abnormalities, FOB with possible endobronchial management seems to be the optimal initial approach (2).
Treatment
Endobronchial therapies
Treatment of massive hemoptysis often begins with instillation of chilled saline or vasoactive agents through flexible or rigid bronchoscopes. Conlan and Hurwitz first described use of cold saline for the management of 12 cases of massive hemoptysis in 1980. Rigid bronchoscopy was used to clear the airway of blood and clot, then to intubate and ventilate of non-bleeding lung. Next, the scope was moved to the mainstem of the bleeding lung. The lung was irrigated with normal saline chilled to 4-degree C in 50-mL aliquots. Suction was applied 30 to 60 seconds after each instillation. Between each lavage, the rigid bronchoscope was returned to the mainstem of the non-bleeding lung for ongoing ventilation, though this is not necessary when using a scope with distal ventilating fenestrations. In all 12 patients, bleeding stopped during the bronchoscopy and lavage. Two patients required repeat bronchoscopy and lavage for rebleeding at three days and 10 days, and one patient had an episode of bradycardia during lavage. Three patients ultimately underwent surgery and several others were started on medical therapy. All were discharged free of hemoptysis (10).
Vasoactive agents, including vasopressin analogs, epinephrine, and norepinephrine, have been used to treat hemoptysis following transbronchial lung biopsies. Doses and dilutions of these agents vary, but doses as low as 0.1 mg of epinephrine have been associated with high drug plasma levels and cardiac arrhythmias (1,2,6). Fortunately, over the past two decades, advances in interventional pulmonology have led to safer alternatives and novel approaches to the endoscopic management of massive hemoptysis. Most of the treatment strategies described below have been introduced in case reports and series and have not yet been validated in large, randomized controlled trials. Many do, however, demonstrate promising results with minimal side effects.
Thermal ablative techniques
Argon plasma coagulation (APC)
APC is a non-contact form of electrocoagulation that allows for rapid coagulation of target tissue. It was first used for bleeding in open surgery, laparoscopy, and in gastrointestinal endoscopy. APC uses electrically conductive gas (argon plasma) to deliver a high-frequency current from a flexible probe to the desired tissue, ablating it and promoting hemostasis. It works on tissues with high water content and low electrical impedance. Once bleeding has stopped, the bronchial wall is less conductive, and deeper tissues are not penetrated (2) (Figure 1).

In 2001, Morice et al. published a review of the efficacy of APC in the bronchoscopic treatment of hemoptysis. The study included 60 patients who underwent 70 FOB procedures to treat hemoptysis and/or neoplastic airway obstruction. Only six patients had severe bleeding of more than 200 mL per day. Bleeding in all cases stopped after APC with no recurrence for a mean of 97±91.9 days (11). Additional investigation is needed to determine if APC is as successful in larger volume hemoptysis. When APC is applied, we recommend forced mode, 20–40 W, 0.8–1.4 LPM via 2.3 mm straight fire, though a pulsed mode also can be utilized.
Electrocautery
Electrocautery is an ablative method used to debulk endobronchial tumors and treat related bleeding. It applies direct electrical energy to the target tissue, producing heat, coagulation, and necrosis. There are no large studies to date describing its use as a bronchoscopic tool in massive hemoptysis (1,2).
Laser therapy
Laser photocoagulation was introduced by Dumon et al. in the early 1980s (12). While most often used to debulk tumors, it also has been applied in the treatment of hemoptysis (13,14). The Nd:YAG and Nd:YAP lasers are most commonly used in bronchoscopy and have wavelengths of 1,060 and 1,340 nm, respectively. The lasers use light energy to produce heat in target tissues, resulting in photocoagulation, vaporization, and necrosis (3). While APC can more rapidly ablate a larger area, laser photocoagulation can penetrate deeper into tissues. Han et al. conducted a retrospective review of laser photocoagulation use in patients with central airway tumor. Of 110 patients, 52 presented with hemoptysis. Following laser treatment, bleeding completely resolved in 77% of patients and partially resolved in 17%. No procedure-related mortality was reported (13). For the Nd:YAG laser, we utilize 20 W/30 Hz with a pulse duration of 0.5–1 as a starting setting.
With each of the ablative methods described above, the patient’s FiO2 must be reduced to 40% prior to use to minimize the risk of airway fire. Therefore, these therapies are not recommended in patients unable to tolerate a decrease in oxygen delivery.
Bronchial-blocking balloon catheters
Balloon catheters have been used for decades to tamponade bleeding in the lung and prevent asphyxiation. Large Foley catheters can be inserted into a mainstem bronchus to isolate a bleeding lung, but smaller transbronchoscopic catheters allow for more targeted blockade of segmental bronchi (2) (Figure 2).
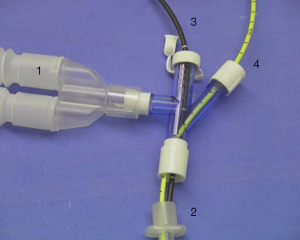
In the early ‘90s, Freitag et al. developed and tested a dual-lumen balloon catheter that could be inserted through the working channel of a fiberoptic bronchoscope. The second lumen was added to allow for ongoing administration of topical agents (2). They utilized 30 catheters in 27 patients over a 36-month period. Underlying diagnoses included malignancy, tuberculosis, aneurysm or vascular deformity, silicosis, and bronchiectasis. All patients had lost at least 100 mL of blood prior to balloon deployment. Blockers were left in place for 15 minutes to 7 days, until more definitive treatment (chemotherapy, radiation, surgery, embolization, antibiotics) could be pursued. Bronchial blockade was successful in all but one case, in which balloon placement was not possible due to tumor location (15).
Silicone spigots
The Endobronchial Watanabe Spigot (EWS) was first used for bronchial occlusion in cases of intractable pneumothorax and bronchopleural fistula (16). It has now been reported as an effective bronchial blocker in cases of massive hemoptysis. The EWS is an endobronchial plug with studs on the outside to prevent migration. It comes in three sizes: small (5 mm), medium (6 mm), and large (7 mm) (17). EWS is most typically employed as a temporizing measure until BAE or surgery can be performed, but there are case reports detailing its use as definitive therapy (18,19).
Sakaguchi et al. recently used an EWS in a patient who was not a candidate for surgery or BAE. The patient was status post aortic valve replacement and coronary artery bypass grafting and developed massive hemoptysis while being weaned from venoarterial extracorporeal membrane oxygenation (ECMO) in the post-operative setting. The bleed was localized to B3, B4, and B5 bronchi via emergent FOB. Three silicone spigots were inserted into each of the bronchi using forceps. Bleeding was controlled, and he was able to be weaned from the vent and from ECMO just days later. The spigots were removed six weeks later, and hemoptysis never recurred (19).
In most reported cases, silicone spigots are inserted into the bronchus using forceps. However, other insertion techniques have been described, including insertion by curette. In a retrospective review, Morikawa et al. studied EWS-based bronchial occlusion procedures performed in 18 consecutive patients. In each case, the bronchoscopist used a Cytology Curette (Olympus Medical Systems) to position the spigot in the target bronchus. Time to EWS occlusion ranged from 65 to 528 seconds, and 93.5% were completed within 5 minutes. All EWS insertions were successful (17). The authors proposed that insertion by curette improves bronchoscopic flexibility and efficiency. Using a curette, the spigot can be easily turned, while forceps can be difficult to navigate into the targeted bronchus (17). Others have raised concern that a silicone spigot inserted by curette may not be able to withstand the pressure of bronchial bleeding and may be difficult, if not impossible, to remove later (20).
It is unclear how long silicone spigots should remain in place once hemostasis is achieved, but, as with any foreign body, they pose a risk of infection. Studies looking at permanent EWS and rates of infection in cases of fistulous lung disease have been conflicting (20). Studies are needed to investigate the risk of infection associated with long-term EWS use.
Endobronchial valves (EBVs)
EBVs were initially designed to reduce lung volume in severe emphysema, but they also have been effectively utilized in the management of bronchopleural fistulas. The one-way endobronchial valve allows air to flow out of a segment of lung during exhalation without allowing air to flow back in during inhalation, leading to atelectasis of an isolated lung segment (21). There are now a handful of published case reports describing successful application of EBVs in persistent or massive hemoptysis.
Lalla et al. recently described a case of massive hemoptysis in a young, HIV-positive male with pulmonary tuberculosis and a left upper lobe (LUL) cavitary lesion. The source of bleeding could not be identified by angiography, and he was deemed a poor surgical candidate. After days of conservative management, massive hemoptysis recurred during a spontaneous breathing trial. On repeat angiography, two abnormal vessels were identified, one of which was embolized. His hemoptysis continued, so the decision was made to intentionally collapse the LUL. A Zephyr EBV was implanted into the LUL bronchus distal to the lingula. Chest X-ray showed an atelectatic LUL with preservation of aeration in the lingula. The patient was extubated 48 hours later with no further hemoptysis. The valve was electively removed at six months with no complications (22).
There are at least three other published cases of EBV use in the management of persistent hemoptysis, however, this appears to be the only case to date describing an application in massive hemoptysis. Proposed mechanisms for the efficacy of EBVs in hemoptysis include the bronchial blockade achieved by the valve itself, the pro-thrombotic effect of the valve, the tamponade effect of lobar atelectasis, and the hypoxic vasoconstriction caused by lobar atelectasis. It is likely some combination of these mechanisms that slows or stops the bleeding (21).
Endobronchial sealing
Endobronchial sealing using biocompatible, quick-drying glue to control hemoptysis has been reported (23-25). In 2002, Bhattacharyya et al. described the use of n-butyl cyanoacrylate glue in six patients with persistent hemoptysis. In each case, FOB was performed under mild sedation, and a polyethylene catheter was passed through the scope’s working channel. Next, 0.5 mL of glue was injected through the catheter with a water column behind. The patients required a total of 0.5 to 1.5 mL of glue to achieve hemostasis. While patients reported expectoration of glue particles in the days following the procedure, no major complications occurred, and no rebleeding was noted during the follow-up period (70 to 250 days) (23).
Coiffard et al. used cyanoacrylate-based glue to occlude the site of bleeding in a patient who had already failed two attempts at BAE and EWS placement. The authors injected 2 mL of glue mixed 50-50 percent with iodinated contrast to facilitate fluoroscopic guidance. A flush of saline was used to advance the glue into sub-segmental bronchi. The bleeding stopped and did not recur during her hospitalization (24).
Ryu et al. applied endobronchial sealing to treat massive hemoptysis resulting from a silicone Y-stent complication. Following airway stabilization and stent removal, the authors placed oxidized regenerated cellulose to achieve hemostasis, then applied polyethylene glycol polymers over top as surgical glue. The bleeding stopped and had not recurred two years after removal of the stent (25).
Endovascular therapies
Bronchial artery embolization (BAE) was introduced as a diagnostic and therapeutic tool for the management of massive hemoptysis in the mid-1970s (26). Since that time, BAE has been routinely used by interventional radiologists as a temporizing measure or as definitive treatment for massive hemoptysis.
First, a descending thoracic aortogram is used to map the bronchial arteries. In CF, ascending aortography or selective subclavian/innominate arteriography may also be needed to identify apical collaterals that have formed over time (4). Bronchial artery selection is performed by injecting iodinated contrast through a 4- or 5-French catheter. Active extravasation of contrast is seen in only 10% to 15% of cases (6,27). Other targets for embolization include tortuous or hypertrophied vessels, hypervascularity, aneurysms, and arteriovenous malformations (AVMs). Next, a “superselective” 3-French microcatheter is inserted through the initial catheter to advance distally and catheterize target vessels. Embolization is performed using gelatin sponge, microspheres, polyvinyl alcohol particles, liquid embolic agents like cyanoacrylate glue, or metallic coils (1,28) (Figure 3).
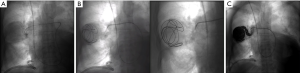
In 2017, Panda et al. published a systematic review of 22 studies reporting on BAE use in hemoptysis. They found that BAE resulted in immediate clinical success (complete cessation of bleeding within 24 hours) 70% to 99% of the time. The preferred embolization agent was polyvinyl alcohol particles (size 300–600 micrometers), a permanent occluding agent. Unfortunately, rebleeding occurred in 10% to 57% of cases. Higher rates of rebleeding were seen in cases of aspergillomas and reactivated or multidrug-resistant tuberculosis (29). Recurrence was attributed to incomplete embolization, recanalization of previously embolized arteries, and development of new collaterals. Risk factors for recurrence included presence of nonbronchial systemic collaterals and bronchopulmonary shunting, which are commonly seen in patients with chronic hypoxia and bronchial inflammation (4).
Han et al. looked specifically at the safety and efficacy of BAE in primary lung cancer-related hemoptysis. In a retrospective review of 84 cases of hemoptysis, technical success, or the ability to successfully embolize the abnormal vessel, was achieved in 98.8% of patients. Clinical success, defined as complete or partial resolution of hemoptysis, was achieved in 82.1% of patients. The median hemoptysis-free survival period for patients was 61 days, however, massive hemoptysis was a risk factor for reduced hemoptysis-free survival post-BAE (HR, 1.83; P=0.12). The authors proposed that massive hemoptysis in patients with lung cancer may be related to neovascularization and new, friable bronchial arteries with higher likelihood of rebleeding (26).
Major complications from BAE are rare with a median incidence of 0.1% (28). These include stroke and spinal cord ischemia from accidental embolization of an anterior medullary spinal artery (27). Additional complications to consider are femoral access site complications and contrast nephropathy.
Tranexamic acid (TXA)
TXA is a synthetic antifibrinolytic agent approved for the treatment or prophylaxis of bleeding in patients with hemophilia and perioperative bleeding associated with major surgeries (3). In these settings, TXA is given orally or intravenously. In 2009, Solomonov et al. published a case series describing the use of topical TXA in six patients with massive hemoptysis. Two patients developed bleeding after biopsy and received a single bolus of 500 mg/5 mL through the working channel of the bronchoscope. The other four bled spontaneously (lung cancer, diffuse alveolar hemorrhage, idiopathic, metastatic thyroid cancer) and received 500 mg/5 mL by inhalation three to four times daily. In all patients, bleeding stopped with the first dose, and no adverse events were reported (30).
Hankerson et al. reported on the use of a nebulized TXA in a patient with an invasive laryngeal tumor. A 10 mg/mL nebulized TXA solution was delivered continuously to his mouth and tracheostomy via facemask. Bleeding stopped within 15 minutes, and the patient experienced no side effects (31).
Recent studies have demonstrated a role for the early use of TXA in trauma patients and in postpartum hemorrhage to decrease mortality (32). A prospective study published in Chest in 2018 also found TXA to be safe and effective in controlling non-massive hemoptysis. Wand et al. conducted a double-blind, randomized controlled trial comparing the use of nebulized TXA (500 mg three times daily) versus placebo (normal saline) in patients admitted with hemoptysis. Patients with massive hemoptysis, defined as more than 200 mL in 24 hours, were excluded. A total of 47 patients were randomized to receive TXA (n=25) or normal saline (n=22). TXA was associated with a significantly reduced volume of expectorated blood by hospital day two, and its use was associated with shorter hospital length of stay and fewer invasive procedures. No side effects were reported (33).
Al-Samkari et al. developed and tested a clinical treatment pathway for systemic antifibrinolytic agent use in CF patients in the inpatient and outpatient setting. Seventy-two episodes of hemoptysis in a total of 21 adult patients with CF were treated according to the pathway. Two-thirds of episodes were considered moderate or massive hemoptysis. Outpatient treatment resulted in a 50% reduction in annual hemoptysis-related admission rate (32).
In studies to date, the use of topical/inhaled TXA for hemoptysis has generally been associated with few adverse events and positive outcomes. However, we recommend its use only in cases of mild to moderate hemoptysis without any imminent threat to airway stability. This includes, for example, patients with mucosal oozing and mild hemoptysis in the setting of thrombocytopenia.
Surgery
Surgical management is an option for most patients with massive hemoptysis, but it is less commonly first-line therapy. Surgery is still the treatment of choice in cases of iatrogenic pulmonary artery rupture, chest trauma, and aspergillomas resistant to other therapies (1). Mortality rates associated with emergency surgery can approach 50% (1,34,35), but rates are significantly lower when surgery is scheduled or planned. Risk factors for poor surgical outcome include advanced age, pleural adhesions, complete pneumonectomy, bronchiectasis, and broncholithiasis (36). The endovascular and endoscopic interventions described above often serve as temporizing measures to bridge to definitive operative management.
Conclusions
Over the past several decades, the management of massive hemoptysis has evolved to include innovative endovascular and endoscopic therapies that, either alone or in conjunction with well-planned surgical interventions, can achieve hemostasis and save lives. We anticipate that the safety and efficacy of these tools and others will be evaluated by large-scale studies as the fields of interventional pulmonology and radiology continue to grow.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Douglas Kyle Hogarth and Jonathan S. Kurman) for the series “Interventional Pulmonology and Advanced Bronchoscopy” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.11.03). The series “Interventional Pulmonology and Advanced Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis 2017;9:S1069-86. [Crossref] [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: An update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Gagnon S, Quigley N, Dutau H, et al. Approach to hemoptysis in the modern era. Can Respir J 2017;2017:1565030 [Crossref] [PubMed]
- Monroe EJ, Pierce DB, Ingraham CR, et al. An interventionalist’s guide to hemoptysis in Cystic Fibrosis. Radiographics 2018;38:624-41. [Crossref] [PubMed]
- Abdulmalak C, Cottenet J, Beltramo G, et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J 2015;46:503-11. [Crossref] [PubMed]
- Yendamuri S. Massive airway hemorrhage. Thorac Surg Clin 2015;25:255-60. [Crossref] [PubMed]
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002;179:1217-24. [Crossref] [PubMed]
- Millar AB, Boothroyd AE, Edwards D, et al. The role of computed tomograph (CT) in the investigation of unexplained haemoptysis. Respir Med 1992;86:39-44. [Crossref] [PubMed]
- Set PA, Flower CD, Smith IE, et al. Hemoptysis: comparative study of the role of CT and fiberoptic bronchoscopy. Radiology 1993;189:677-80. [Crossref] [PubMed]
- Conlan AA, Hurwitz SS. Management of massive hemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-4. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Dumon JF, Reboud E, Garbe L, et al. Treatment of tracheobronchial lesions by laser photoresection. Chest 1982;81:278-84. [Crossref] [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [Crossref] [PubMed]
- Sheth HS, Maldonado F, Lentz RJ. Two cases of Dieulafoy lesions of the bronchus with novel comorbid associations and endobronchial ablative management. Medicine 2018;97:e9754 [Crossref] [PubMed]
- Freitag L, Tekolf E, Stamatis G, et al. Three years experience with a new balloon catheter for the management of haemoptysis. Eur Respir J 1994;7:2033-7. [PubMed]
- Watanabe Y, Matsuo K, Tamaoki A, et al. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchol 2003;10:264-7. [Crossref]
- Morikawa S, Okamura T, Minezawa T, et al. A simple method of bronchial occlusion with silicone spigots (Endobronchial Watanabe Spigot; EWS) using a curette. Ther Adv Respir Dis 2016;10:518-24. [Crossref] [PubMed]
- Hozumi T, Kajiura K, Nakamura K, et al. Aorto-pleural fistula successfully treated by one-lung ventilation and Endobronchial Watanabe Spigots. Respirol Case Rep 2018;7:e00382 [Crossref] [PubMed]
- Sakaguchi T, Kida H, Kanno Y, et al. Bronchial occlusion with Endobronchial Watanabe Spigot for hemoptysis in a mechanically ventilated patient with extracorporeal circulation. Intern Med 2019;58:267-9. [Crossref] [PubMed]
- Adachi T, Oki M, Saka H. Management considerations for the treatment of idiopathic massive hemoptysis with endobronchial occlusion combined with bronchial artery embolization. Intern Med 2016;55:173-7. [Crossref] [PubMed]
- Patel B, Abi-Fadel D, Rosenheck J, et al. Endobronchial valves for treatment of hemoptysis. J Bronchology Interv Pulmonol 2019;26:e22-4. [Crossref] [PubMed]
- Lalla U, Allwood B, Roy SS, et al. Endobronchial valve used as salvage therapy in a mechanically ventilated patient with intractable life-threatening haemoptysis. Respiration 2017;93:436-40. [Crossref] [PubMed]
- Bhattacharyya P, Dutta A, Samanta AN, et al. New procedure: Bronchoscopic endobronchial sealing: A new mode of managing hemoptysis. Chest 2002;121:2066-9. [Crossref] [PubMed]
- Coiffard B, Dutau H, Laroumagne S, et al. Endobronchial sealing with glue for malignant hemoptysis. J Bronchology Interv Pulmonol 2014;21:373-5. [Crossref] [PubMed]
- Ryu C, Boffa D, Bramley K, et al. A novel endobronchial approach to massive hemoptysis complicating silicone Y-stent placement for tracheobronchomalacia: a case report. Medicine 2018;97:e9980 [Crossref] [PubMed]
- Han K, Yoon KW, Kim JH, et al. Bronchial artery embolization for hemoptysis in primary lung cancer: A retrospective review of 84 patients. J Vasc Interv Radiol 2019;30:428-34. [Crossref] [PubMed]
- Gupta A, Sands M, Chauhan NR. Massive hemoptysis in pulmonary infections: bronchial artery embolization. J Thorac Dis 2018;10:S3458-64. [Crossref] [PubMed]
- Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: A comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol 2010;33:240-50. [Crossref] [PubMed]
- Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017;23:307-17. [Crossref] [PubMed]
- Solomonov A, Fruchter O, Zukerman T, et al. Pulmonary hemorrhage: A novel mode of therapy. Respir Med 2009;103:1196-200. [Crossref] [PubMed]
- Hankerson MJ, Raffetto B, Mallon WK, et al. Nebulized tranexamic acid as a noninvasive therapy for cancer-related hemoptysis. J Palliat Med 2015;18:1060-2. [Crossref] [PubMed]
- Al-Samkari H, Shin K, Cardoni L, et al. Antifibrinolytic agents for hemoptysis management in adults with Cystic Fibrosis. Chest 2019;155:1226-33. [Crossref] [PubMed]
- Wand O, Guber E, Guber A, et al. Inhaled tranexamic acid for hemoptysis treatment. Chest 2018;154:1379-84. [Crossref] [PubMed]
- Kiral H, Evman S, Tezel C, et al. Pulmonary resection in the treatment of life-threatening hemoptysis. Ann Thorac Cardiovasc Surg 2015;21:125-31. [Crossref] [PubMed]
- Andréjak C, Parrot A, Bazelly B, et al. Surgical lung resection for severe hemoptysis. Ann Thorac Surg 2009;88:1556-65. [Crossref] [PubMed]
- Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-9. [Crossref] [PubMed]
Cite this article as: Cook CM, Jasahui MP. A review of the modern, multidisciplinary approach to the management of massive hemoptysis. Shanghai Chest 2020;4:5.


