Pneumonectomy in pulmonary tuberculosis—to do or not to do?
Introduction
Tuberculosis is the only infectious disease where surgical methods are applied for the treatment. Surgical interventions for pulmonary tuberculosis (PTB) are used to remove anatomically irreversible alterations namely lung cavities and destroyed lung. The most challenging issue here is an extremely high rate of drug resistance in cavitary PTB. Cavitation is the most typical manifestation of multi-drug resistant (MDR) PTB (40–80%) (1). Lung cavity contains a great number of mycobacteria tuberculosis (MBT), and antibiotic penetration into the cavity is very difficult (2,3). The treatment of such patients is very difficult because of severity of PTB, drug resistance of MBT, and toxic effects of previous chemotherapy. Pneumonectomy (PE) often remains the only chance for recovery in PTB patient with a destroyed lung where therapy is powerless (3). Obvious problems in preparing these patients for the operation, and a high risk of postoperative complications often hinder the surgical activeness. Hence it is clear that the most representative series of publications on pneumonectomies are devoted to the problems of MDR and extensively drug resistant (XDR) PTB (2-7). The aim of this study is to analyze our own experience and review the available literature on this issue.
Methods
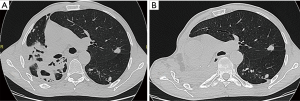
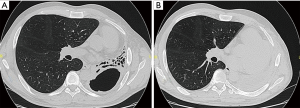
The cases of 64 patients with PTB who underwent PE during last 10 years were reviewed. All the patients were with failure of previous treatment. The male/female ratio was 36/28. The age range was from 21 to 59 years. The average age was 44.7 years. The body mass index lower than 18.5 was in 27 patients (42.2%). In all cases there was cavitary disease with spread over the whole lung. Empyema complicated the disease in 10 cases, haemoptysis in 9 cases. There were 6 completion PE, Figure 1. Drug resistance was observed in all patients including MDR in 40 (62.5%), and XDR in 20 (31.3%). The degree of drug resistance was estimated by at least two repeated examinations of culture patterns. Right PE was performed in 30 patients and left in 34. A picture of right PE is presented on the Figure 2. All patients had received individual multiple-drug chemotherapy according to drug susceptibility test results. The therapy was conducted in accordance with the WHO guidelines (8). The course of antibiotics to suppress the activity of non-specific microorganisms was also carried out.
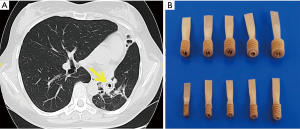
Collapse therapy was administered as a supplementary treatment in 36 patients (Table 1). The main purpose of collapse therapy was to reduce the cavity size and pericavitary infiltration. The indications to collapse therapy were based on the CT scan picture and particular importance was attached to the presence of infiltration and foci around the cavity found at CT scan. Artificial pneumothorax (AP) was applied before the operation in 14 patients with lesions localized in the upper lobe or apical segment of the lower lobe. Endobronchial valve (EBV) treatment was applied in 13 patients (Figure 3) where lung cavities were localized in the middle or lower lobe, and in the cases of AP failure mostly because of wide pleural adhesions. In cases of bilateral cavitary disease EBV was used on both sides, while AP was applied only on the opposite side. AP and/or EBV were combined with pneumoperitoneum (PP) in cases of dissemination in the lower lobes.
Table 1
| Procedure | No. of patients |
|---|---|
| AP gomolateral | 6 |
| AP opposite | 8 |
| EBV | 11 |
| AP + EBV | 2 |
| PP | 9 |
| Total | 36 |
AP, artificial pneumothorax; EBV, endobronchial valve; PP, pneumoperitoneum.
In cases of endobronchitis we administered treatment which included nebulized anticholinergic and mucolytic agents combined with local endobronchial lavage. This facilitated bronchial drainage of the cavity. A complex of measures was also undertaken to reduce the tuberculous activity and improve the general conditions of patients. The said complex comprised: (I) enteral and parenteral supplementation in an attempt to obtain a positive nitrogen balance; (II) treatment of concomitant illnesses and correction of toxic effects of chemotherapy (III) therapy for improving immunity and respiratory status. The patients were subjected the aforementioned regimen of the treatment on average for 2–3 months. The appropriate timing for the surgery was considered to be when the general condition of the patient was improved as much as possible, and temporary sputum conversion or at least maximal reduction of MBT expectoration had been achieved. Thorough endoscopic inspection and endobronchial biopsy was carried out before the operation to make sure that the main bronchus was not affected by tuberculosis. If so, a special treatment namely endobronchial instillation of drugs was undertaken. The written informed consent for treatment and data publication and any accompanying images was taken in all the cases.
All operations were performed via lateral thoracotomy. Dense pleural adhesions were divided with the help of electrocautery and/or argon beam coagulator in order to control bleeding and prevent cavity damage during extrapleural dissection. Bronchial stump was closed by stapling and by manual suturing in 25, and in 39 patients respectively. The stump of the main bronchus was reinforced with an intercostal muscle flap (n=19), pericardial fat pad and mediastinal pleura (n=33), and with a latissimus dorsi muscle flap (n=12). Filling of the pleural cavity was under constant supervision by punctures and the fluid remained under laboratory control until three consecutive negative cultures were obtained. The removed lungs were subjected to bacteriologic investigation in order to specify the bacterial spectrum. The length of patients stay in the thoracic surgery department depended on the postoperative course. In uneventful cases it was about 7–10 days. After that the patients were transferred back to the MDR department for continuation of the therapy, which was generally the same as before the operation. Supervision of the thoracic surgeon was provided when necessary.
Duration of the continuation phase therapy after surgery was 16–24 months. The regimen was mainly the same as before but in some cases it was adjusted after bacteriologic assessment of the removed specimen. The patients who had completed the treatment continued to be under the supervision of the local dispensary for at least 1 year.
Results
Sputum conversion before the operation was achieved in 27 (42.2%) patients. There was one intraoperative complication—injury of the left subclavian artery healed by vascular suturing. No other complications occurred in this patient. Postoperative major complications were in 20 patients (31.3%) (Table 2).
Table 2
| Complications | Right side (n=30) | Left side (n=34) | Total (n=64) | Mortality |
|---|---|---|---|---|
| Bleeding, n (%) | 5 (16.7) | 3 (8.8) | 8 (12.5) | – |
| Respiratory failure, n (%) | 2 (6.7) | 1 (2.9) | 3 (4.7) | – |
| BPF and empyema, n (%) | 5 (16.7) | 4 (11.8) | 9 (14.1) | 4 (44.4) |
| Total, n (%) | 12 (40.0) | 8 (23.5) | 20 (31.3) | 4 (6.2) |
BPF, bronchopleural fistulas.
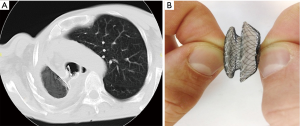
Postoperative intrapleural bleeding was in 8 patients. The source of bleeding was the pleural surface after pneumolysis. Rethoracotomy became necessary in 4 of them. Bronchopleural fistulas (BPF) and empyema took place in 9 (14.1%) cases. Three patients with BPF were operated on in emergency situations when the serious haemoptysis took place. These 3 patients were also found to have bronchial tuberculosis at the bronchial resection line. All patients with postoperative BPF had MBT+ before the operation (P<0.05). The period from the operation to BPF development was from 3 weeks to 2 months (average 27 days). In 3 patients BPF were healed simultaneously with PTB regression in the opposite lung, and in 6 cases occlusion of BPF with Amplatzer atrial septal defect (ASD) occluder was performed, Figure 4. In 5 cases this procedure was successful, and 1 patient died from PTB progression after violating the treatment regimen. No relationship between the side of the operation, mode of bronchial stump closing and the BPF and empyema rates was observed. Hospital mortality rate was 6.2%. All postoperative deaths resulted from BPF and empyema in patients with PTB progression.
Sputum culture conversion was achieved in all 61 patients who survived the operation. Ten patients are still under therapy according to the treatment protocols. Ten (16.4%) patients relapsed, with MBT expectoration, and they are also under treatment. Five of them died from the disease. All cases of relapses were resulted from the treatment regimen violating. Forty-one (67.2%) patients remain free of the disease including those passed through postoperative complications.
Discussion
The history of PE in PTB goes back to 1897 when Macewen performed the first successful left PE for tuberculosis. The patient was healthy 14 years after surgery. This fact is mentioned in Garrè’s and Quincke’s monograph (9), but it’s not entirely clear whether this was actually a PE. A surgical technique of PE with intrapleural lung dissection and individual ligation of the pulmonary great vessels and closure of the bronchus was implemented and described by Rienhoff in 1936 (10). Now PE is performed in patients with cavitary MDR/XDR tuberculosis (TB) and destroyed lung. A destroyed lung is formed as a result of a prolonged infectious process, when bacteria develop drug resistance, and the patient’s protective reserves become significantly depleted. This fact explains high rates of PE in the series published in the literature devoted to the treatment of drug-resistant PTB (Table 3).
Table 3
| Author | Year | No. of operations | No. of PE | Rate of PE, % |
|---|---|---|---|---|
| van Leuven (11) | 1997 | 62 | 35 | 56.4 |
| Pomerantz (2) | 2001 | 180 | 82 | 45.5 |
| Chiang (12) | 2001 | 27 | 10 | 37.0 |
| Shiraishi (7) | 2004 | 33 | 12 | 36.4 |
| Naidoo (13) | 2005 | 27 | 17 | 62.9 |
| Kir (6) | 2006 | 81 | 39 | 48.1 |
| Mohsen (14) | 2007 | 23 | 11 | 47.8 |
| Somocurcio (15) | 2007 | 121 | 27 | 22.3 |
| Wang (16) | 2008 | 56 | 25 | 44.6 |
| Kang (4) | 2010 | 72 | 23 | 32.0 |
| Bai (17) | 2012 | 176 | 149 | 84.6 |
| Vashakidze (18) | 2013 | 75 | 8 | 10.7 |
| Xie (1) | 2013 | 43 | 8 | 18.6 |
| Marfina (19) | 2018 | 57 | 28 | 49.1 |
PE, pneumonectomy.
The complexity of the problem is as follows. Patients with destroyed lungs develop profound immune disorders. The vast majority of them have MDR/XDR TB. These patients suffer by toxic effects of chemotherapy, and experienced failures of at least one previous course of therapy (19,20). The treatment is based on individualized multiple-drug chemotherapy as determined by drug susceptibility studies (1,2,6,7,19,20). The criteria of the patient’s preparedness for surgery are: stabilization of the tuberculosis process; resorption or densification of foci of seeding; normalization of the haemogram and biochemical parameters of blood (20). Sputum negativation or, at least, the maximum decrease in the intensity of bacterial expectoration is considered to be a critical prognostic factor both in terms of prevention of postoperative complications, as well as for long-term prognosis. A favorable moment for the operation is created in this way 2–3 months after the start of a proper regimen of the therapy (1,2,4,6,14). This is not always possible to achieve, and the operation has to be performed with continuing bacterial excretion including the cases when the operation is indicated for urgent reasons, in particular, bleeding which seriously worsens the prognosis (5,12,17).
Malnutrition (body mass index <18.5) was registered in 19.3% (19), 30% (15), 99% (2) in these groups of patients and was also a serious problem (2,15,20). Attempts to improve nutritive status and obtain a positive nitrogen balance are often unsuccessful due to severity of the disease (2).
All of the above accounts for the fact that PE for PTB is accompanied by a rather high frequency of postoperative complications and mortality (2,4,5,7,16,21).
The data available in literature (2,16,21) and our data showed that postoperative complications in PTB surgery are a problem especially serious after PE. The most crucial problems are BPF and empyema. The rates of BPF after PE are presented in the Table 4. The question about causes of this complication, its prevention and treatment still remain a subject of discussion. We found no relationship between mode of bronchial stump closing and BPF rate after PE. A similar conclusion was reached by other authors (5-7,13,16,18,21). It is recommended to cover the bronchial stump in order to prevent BPF (2,6,7,15,16,22-24). Some methods are applied here, namely, pleura, pericardial fat pad, pericardial flap, intercostal muscle, but a latissimus dorsi muscles considered preferable (2,6,7). In our series no BPF occurred after covering the stump by this muscle. On the other hand, some authors do not consider covering the stump obligatory (5,13,18). However, our experience in surgery, not only for tuberculosis, but also for lung cancer, convinced us of the need to buttress the stump. All bronchopleural complications in our study were connected with PTB reactivation. These processes are interrelated and seriously aggravate each other. In the multivariate analysis performed by Kim et al. (5) was showed that, low preoperative FEV1 (P=0.006), postoperative positive sputum MBT (P=0.019) and the presence of aspergilloma (P=0.024) were significant risk factors for the development of postoperative BPF. Wang et al. (16) came to the similar conclusion. Hence, the prevailing opinion is that the main cause of postoperative complications, namely BPF, is activity of tuberculosis at the time of operation: persistent bacilli expectoration, MDR, and tuberculosis at the bronchial resection line (2,5,7,16,21,25,26). Wang (16) stressed that for preventing postpneumonectomy BPF, it is reasonable to perform frozen section pathology on the resected bronchial stump to make sure that no MBT remains in the bronchial stump. A thorough assessment of the condition of the bronchus before the operation is more important for preventing fistulas (24). We share that opinion but would like to note that the affected bronchial stump can be found where PE is performed in urgent situations namely in cases of bleeding. In our series the vast majority of BPF developed in patients operated on in active stage of the disease with continuous MBT expectoration.
Table 4
| Author | Year | No. of PE | No. of BPF | %% |
|---|---|---|---|---|
| Massard (22) | 1996 | 25 | 3 | 12 |
| van Leuven (11) | 1997 | 35 | 2 | 5.7 |
| Pomerantz (2) | 2001 | 82 | 5 | 6.1 |
| Kim (5) | 2003 | 94 | 7 | 7.5 |
| Shiraishi (7) | 2004 | 12 | 1 | 8.3 |
| Naidoo (13) | 2005 | 17 | 1 | 5.9 |
| Kir (6) | 2006 | 43 | 3 | 7.0 |
| Somocurcio (15) | 2007 | 27 | 5 | 18.5 |
| Mohsen (14) | 2007 | 11 | – | – |
| Wang (16) | 2008 | 25 | 4 | 16 |
| Ots (20) | 2009 | 129 | 19 | 14.7 |
| Kang (4) | 2010 | 23 | – | – |
| Bai (17) | 2012 | 147 | 3 | 2.0 |
| Marfina (19) | 2018 | 28 | 4 | 14.3 |
| Harmouchi (23) | 2019 | 38 | 2 | 5.3 |
BPF, bronchopleural fistulas; PE, pneumonectomy.
In this regard Shiraishi (7) believed that the development of bronchial fistulas was not caused by any problems with the surgical technique. In their study late BPF were preceded by a TB relapse at the bronchial stump. The results of our study led us to the same conclusion. Thus, it is considered that even excellent performance the PE does not guarantee a favorable outcome of the treatment in patients with a high degree of the TB infection activity. Significant efforts are required to reduce the risk of complications and to treat them if they do occur (7,22). Consequently, PE should not be considered a subject of choice, but rather a forced measure (2,4,7). No mortality after PE in TB was reported in following studies (1,16,18,25). Other authors present mortality rates, which can be regarded as acceptable taking into account the complexity of the patients who are operated on: 1.1% (5); 3.4% (17); 3.9% (20); 4% (22); 4.3% (4); 4.6% (6); 5.2% (23); 5.3% (27); 9.1% (14).
In cases of bilateral cavitary disease staged resections are indicated if the patient can tolerate bilateral surgery (5-7,19,28). In the study by Marfina et al. (19) 13 patients with MDR/XDR TB patients who underwent PE were subjected to delayed contralateral selective thoracoplasty (n=10) or resection (n=3) without postoperative mortality. Sputum culture conversion was achieved in 78,6% of these patients.
Duration of the continuation phase therapy after surgery is recommended to be 12–24 months mainly with the same regimens as before the operation (2,4,7,28). In some cases correction of the therapy is necessary after bacteriologic investigation of the resected specimen. Kang et al. (4) considered continuation of the therapy necessary for at least 12 months after culture conversion. Under these conditions, a good result is achieved by Chiang et al. —up to 92% of sputum conversion (12).
The most frequent underlying disease in patients with pulmonary aspergillosis (so called “fungus ball”) was tuberculosis, especially in those infected by complex aspergilloma 61.5% (29), 65% (30), 69% (5), 72.3% (24), 89% (25). We do not have our own PE experience regarding pulmonary aspergillosis. As we can see from the literature available to us, PE for aspergillosis is performed in the following cases: (I) the affected lung is totally destroyed; (II) the remaining lobe is fibrotic and small and there is a high risk of residual cavity formation; and (III) completion PE (22,24,31,32). The most characteristic complication of aspergillosis requiring surgical intervention is recurrent bleeding, which in some cases takes on a fatal character (24,25,29,32,33). It is emphasized that even asymptomatic aspergillomas are subject to surgical treatment, since the risk of bleeding is always rather high (22,24,25). Preoperatively bronchial artery embolization in cases of major hemoptysis was performed to temporarily control bleeding and prevent life-threatening situation (24,25,30,32). Significant intraoperative technical difficulties in the surgery of aspergillosis are mainly caused by long underlying diseases and result in the obliterated pleural space and indurated hilar structures. Meticulous extrapleural pneumolysis is necessary to dissect dense vascular adhesions and to avoid injury of infected cavities (4,5,22,24,31,32). Bronchial stump reinforcement with the muscle flap or other well vascularised tissue is strongly recommended (22,24,31,32). In general, aspergillosis surgery is based on the same principles as surgery for other infectious lung diseases. Kim et al. (5) found the presence of aspergilloma was a significant risk factor for the development of postoperative BPF after PE in TB patients. On the other hand, Massard et al. (22) consider that aspergillus disease in patients with previous tuberculosis did not increase the risk of postoperative complications. Shiraishi et al. (32) presented the data on two complications in 11 pneumonectomies in patients with aspergillosis without mortality. There was no statistically significant difference in the postoperative morbidity between patients infected simple- and complex aspergillus (P=0.27) (24). There is the data in the literature on 69 pneumonectomies in aspergillosis with 3 deaths (4.3%) (4,5,24,29-35), and this rate can be considered acceptable. Hence, aggressive surgical resection for pulmonary aspergilloma in both symptomatic and asymptomatic patients is approved especially given a high risk of bleeding in such patients.
Conclusions
It should be emphasized once again that PE for tuberculosis is rather a forced measure. A very high risk of this operation is justified by the fact that for a number of patients this is the only chance for recovery. PE can be performed with acceptable morbidity and mortality and can achieve satisfactory long-term survival. Special care is recommended to prepare patients to the operation especially for preventing postoperative bronchopleural complications. Adherence to the treatment is a necessary requirement for achieving positive results.
Acknowledgments
The authors are indebted to clinical staff for a great help in the treatment of our patients. Authors are also appreciated to their families for the patience and understood.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.10.01). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Local Ethical Committee protocol #25 from 10.09.2019 and individual consent for this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie B, Yang Y, He W, et al. Pulmonary resection in the treatment of 43 patients with well-localized, cavitary pulmonary multidrug-resistant tuberculosis in Shanghai. Interact Cardiovasc Thorac Surg 2013;17:455-9. [Crossref] [PubMed]
- Pomerantz BJ, Cleveland JC Jr, Olson HK, et al. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 2001;121:448-53. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Different morbidity after pneumonectomy: multidrug-resistant tuberculosis versus non-tuberculous mycobacterial infection. Interact Cardiovasc Thorac Surg 2010;11:429-32. [Crossref] [PubMed]
- Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [Crossref] [PubMed]
- Kim YT, Kang MC, Sung SW, et al. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294-8. [Crossref] [PubMed]
- Kir A, Inci I, Torun T, et al. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2006;131:693-6. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2004;128:523-8. [Crossref] [PubMed]
- Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Geneva: World Health Organization, 2011:516-28.
- Garrè C, Quincke H. Lungenchirurgie. Jena: Gustav Fischer, 1912:158.
- Rienhoff WF. The Surgical technic of total pneumonectomy. Arch Surg 1936;32:218-31. [Crossref]
- van Leuven M, De Groot M, Shean KP, et al. Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann Thorac Surg 1997;63:1368-72; discussion 1372-3. [Crossref] [PubMed]
- Chiang CY, Yu MC, Bai KJ, et al. Pulmonary resection in the treatment of patients with pulmonary multidrug-resistant tuberculosis in Taiwan. Int J Tuberc Lung Dis 2001;5:272-7. [PubMed]
- Naidoo R, Reddi A. Lung resection for multidrug-resistant tuberculosis. Asian Cardiovasc Thorac Ann 2005;13:172-4. [Crossref] [PubMed]
- Mohsen T, Zeid AA, Haj-Yahia S. Lobectomy or pneumonectomy for multidrug-resistant pulmonary tuberculosis can be performed with acceptable morbidity and mortality: a seven-year review of a single institution's experience. J Thorac Cardiovasc Surg 2007;134:194-8. [Crossref] [PubMed]
- Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax 2007;62:416-21. [Crossref] [PubMed]
- Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg 2008;86:1640-5. [Crossref] [PubMed]
- Bai L, Hong Z, Gong C, et al. Surgical treatment efficacy in 172 cases of tuberculosis-destroyed lungs. Eur J Cardiothorac Surg 2012;41:335-40. [Crossref] [PubMed]
- Vashakidze S, Gogishvili S, Nikolaishvili K, et al. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg 2013;95:1892-8. [Crossref] [PubMed]
- Marfina GY, Vladimirov KB, Avetisian AO, et al. Bilateral cavitary multidrug- or extensively drug-resistant tuberculosis: role of surgery. Eur J Cardiothorac Surg 2018;53:618-24. [Crossref] [PubMed]
- Ots ON, Agkatsev TV, Perel'man MI. Surgical treatment for pulmonary tuberculosis with mycobacterium resistant to drugs. Probl Tub 2009;2:42-9.
- Gimferrer JM, Mestres CA. Role of surgery in drug-resistant pulmonary tuberculosis. Asian Cardiovasc Thorac Ann 2005;13:201-2. [Crossref] [PubMed]
- Massard G, Dabbagh A, Wihlm JM, et al. Pneumonectomy for chronic infection is a high-risk procedure. Ann Thorac Surg 1996;62:1033-7; discussion 1037-8. [Crossref] [PubMed]
- Harmouchi H, Sani R, Belliraj L, et al. Pneumonectomy for non-tumoral diseases: etiologies and follow-up in 38 cases. Asian Cardiovasc Thorac Ann 2019;27:298-301. [Crossref] [PubMed]
- Chen QK, Jiang GN, Ding JA. Surgical treatment for pulmonary aspergilloma: a 35-year experience in the Chinese population. Interact Cardiovasc Thorac Surg 2012;15:77-80. [Crossref] [PubMed]
- Park SK, Lee CM, Heu JP, et al. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2002;6:143-9. [PubMed]
- El'kin AV, Repin IuM, Levashev IuN. Long-term results of surgical treatment of pulmonary tuberculosis wtih respect to the extent of bacterial isolation and to drug resistance of Mycobacterium tuberculosis. Probl Tuberk Bolezn Legk 2003;28-31. [PubMed]
- Souilamas R, Riquet M, Barthes FP, et al. Surgical treatment of active and sequelar forms of pulmonary tuberculosis. Ann Thorac Surg 2001;71:443-7. [Crossref] [PubMed]
- Subotic D, Yablonskiy P, Sulis G, et al. Surgery and pleuro-pulmonary tuberculosis: a scientific literature review. J Thorac Dis 2016;8:E474-85. [Crossref] [PubMed]
- Citak N, Sayar A, Metin M. Pulmoner aspergillomada altı yıllık 26 olguluk cerrahi tedavi sonuçları: tek cerrahi servisinin deneyimi. Tüberküloz ve Toraks Dergisi 2011;59:62-9. [Crossref]
- Regnard JF, Icard P, Nicolosi M, et al. Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg 2000;69:898-903. [Crossref] [PubMed]
- Al-Kattan K, Ashour M, Hajjar W, et al. Surgery for pulmonary aspergilloma in post-tuberculous vs. immuno-compromised patients. Eur J Cardiothorac Surg 2001;20:728-33. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Nakajima Y, et al. Pneumonectomy for complex aspergilloma: is it still dangerous? Eur J Cardiothorac Surg 2006;29:9-13. [Crossref] [PubMed]
- Lejay A, Falcoz PE, Santelmo N, et al. Surgery for aspergilloma: time trend towards improved results? Interact Cardiovasc Thorac Surg 2011;13:392-5. [Crossref] [PubMed]
- Ba PS, Ndiaye A, Diatta S, et al. Results of surgical treatment for pulmonary aspergilloma. Med Sante Trop 2015;25:92-6. [Crossref] [PubMed]
- Babatasi G, Massetti M, Chapelier A, et al. Surgical treatment of pulmonary aspergilloma: current outcome. J Thorac Cardiovasc Surg 2000;119:906-12. [Crossref] [PubMed]
Cite this article as: Motus IY, Bazhenov AV. Pneumonectomy in pulmonary tuberculosis—to do or not to do? Shanghai Chest 2020;4:10.