
Carinal pneumonectomy: a 36-year experience
Introduction
Tracheal sleeve pneumonectomy (TSP) or carinal pneumonectomy, is a rare and challenging procedure.
Its main indications are non-small cell lung cancer (NSCLC) extending up to the carina or tracheobronchial angle, or rarer tumours of the central airway, such as carcinoid tumours or adenoid cystic carcinoma (ACC). The operation usually consists in a right TSP, while left TSP is extremely rare. In fact, the left main bronchus is longer than the right one, and tumours at this level frequently invade the subaortic space (1). Technical difficulties include intraoperative ventilation management and the dissection, resection and reconstruction of the carina and main bronchi, which are structures located deep into the mediastinum. Morbidity and mortality are higher than those reported after standard pneumonectomy [11% to 50% and 3% to 20%, respectively (2)], and they are primarily due to acute respiratory distress syndrome (ARDS) and anastomotic complications. The most important unfavourable prognostic factor for long-term survival is pathologic N2 disease, which leads to a 5-year overall survival (OS) rate of 12%, as compared to 51% in N0 patients (3).
We previously presented our experience with TSP for NSCLC, based on 49 cases from 1982 to 2005 (4). With the present paper, we want to provide an update of the indications, techniques and outcomes of TSP at our Institution, including all operated patients over a time span of 36 years.
Methods
Patient data
Medical records of 63 consecutive patients undergoing TSP at the Thoracic Surgery Division of Padua University Hospital between January 1982 and December 2018 were retrospectively reviewed. Pneumonectomies associated with only partial, non-circumferential tracheo-carinal resections or isolated carinal resections were not included. Data were extracted from a prospectively maintained database and included patients’ demographics, medical history, disease presentation, prior treatments, operative report, morbidity and mortality, hospital stay, postoperative results and final pathology report. Follow-up was obtained by consultation of medical records from outpatient visits or, where missing, by telephone interview with the patient or his/her relatives. Patients with missing data concerning one or more of the independent variables, or those with incomplete follow-up information were excluded from the analysis. Written informed consent was obtained from all patients and the study was approved by the institutional review board.
Overall, 57 men and 6 women, with a median age of 61 years (range, 37–79 years) were included. Main characteristics of the study cohort are summarized in Table 1. The majority of patients (n=59) underwent right TSP, while 4 patients underwent left TSP. In 8 cases, right TSP was associated with resection of the superior vena cava (SVC), including 6 partial and 2 total resections. Following partial resections, the SVC was either directly sutured (n=3), or reconstructed with a pericardial patch (n=3), while in cases of more extensive involvement, it was completely resected and substituted using a ringed polytetrafluoroethylene (PTFE) graft.
Table 1
| Variable | Values |
|---|---|
| Age (years) | |
| Median | 61 |
| Range | 37–79 |
| Gender | |
| Male | 57 |
| Female | 6 |
| Histology | |
| Squamous cell carcinoma | 44 |
| Adenocarcinoma | 10 |
| ACC | 7 |
| Others* | 2 |
| Preoperative treatment | |
| Radiotherapy | 11 |
| Chemotherapy | 9 |
| Chemo-radiotherapy | 3 |
| Operative side | |
| Right | 59 |
| Left | 4 |
| Ventilation management | |
| HFJV | 53 |
| Cross-field intubation | 5 |
| Extra-long ET | 3 |
| Extracorporeal circulation | 2 |
| SVC resection | |
| Partial | 6 |
| Complete | 2 |
| Type of flap coverage | |
| None | 21 |
| Pericardium | 24 |
| Intercostal muscle | 14 |
| Others** | 4 |
| Pathologic T stage | |
| T3 | 29 |
| T4 | 33 |
| Pathologic N stage | |
| N0 | 22 |
| N1 | 22 |
| N2 | 18 |
| N3 | 1 |
| Resection margins | |
| R0 | 52 |
| R1 | 11 |
*, large-cell carcinoma (n=1) and small-cell carcinoma (n=1); **, parietal pleura (n=2), mammary artery (n=1), and azygos vein (n=1). ET, endotracheal tube.
Given the long study period, patients were pre-operatively treated according to different multimodality protocols. In total, 23 patients underwent neoadjuvant therapy, including radiotherapy (n=11, between 1982 and 1995), chemotherapy (n=9), and chemo-radiotherapy (n=3).
Preoperative workup
All patients scheduled to undergo sleeve pneumonectomy underwent a careful functional evaluation as previously described (4). Briefly, spirometry, arterial blood gas analysis, electrocardiography and transthoracic echocardiography where performed in all patients, and a predicted post-operative FEV1 greater than 1L, as determined by ventilation-perfusion scan, was used as a cut-off to determine functional operability. In more recent years (approximately from 2000), cardiopulmonary exercise testing was consistently added to the preoperative workup, in order to better stratify the risk of major cardiopulmonary complications (5). For patients affected by NSCLC, preoperative staging consisted of a computed tomography (CT) of the chest, upper abdomen and brain, flexible bronchoscopy, and positron emission tomography (PET) scan (only starting from year 2000). Mediastinoscopy was performed in cases of enlarged or PET-positive mediastinal lymph nodes. Patients with histologically proven N2 disease were treated with induction chemotherapy and reassessed with a repeat PET scan: responders or those with stable disease were scheduled for surgery.
Surgical technique
The surgical technique of carinal pneumonectomy has already been detailed in multiple papers and reviews (2,6,7) as well as in our previous report (4). Some specific aspects are underlined here.
An epidural catheter is placed to provide postoperative analgesia and patients are initially ventilated through a double-lumen endotracheal tube. Rigid bronchoscopy is performed at the time of surgery or shortly before it, in order to precisely assess the airway and plan the procedure.
Right TSP is performed through a thoracotomy in the 4th intercostal space. Azygos vein is divided and the tracheobronchial bifurcation is mobilized. Circumferential dissection is limited to no more than 2 cm from the proposed lines of transection of the lower trachea and the left main bronchus, while the pretracheal plane is systematically developed as a release manoeuvre. For left TSP, on the other hand, due to the rarity of the procedure, no single standard incision is recommended. Over time, we used different approaches, including left thoracotomy, “clamshell incision”, median sternotomy and, more recently, right thoracotomy combined with a left thoracoscopy.
After removal of the specimen, resection margins are sent for frozen section examination. Then, end-to-end anastomosis begins with three interrupted 3-0 Vicryl stitches placed on the deepest part at the level of the left membranous-cartilaginous angle. At this point the endotracheal tube is withdrawn into the trachea and ventilation is provided according to one of these three modalities: cross-field intubation, high-frequency jet ventilation (HFJV), or an extra-long single-lumen endotracheal tube (PHYCON Wire Reinforced Endotracheal Tube, inner diameter: 6.5 mm, 400 mm long type, Fuji Systems Corporation, Tokyo, Japan), with a small distal cuff, which allows placement of the sutures (Figure 1). Generally, all of the aforementioned techniques for intraoperative ventilation management are effective. Extracorporeal circulation is a fallback solution for particular cases, were dissection or exposure are more difficult. Several interrupted 3-0 Vicryl stitches are placed in the remaining part of the anastomosis and are tied after all of them have been placed to correct for size discrepancies. Before tying the knots, the neck is flexed moderately, to reduce tension on the anastomosis, and the patient is ventilated with the endotracheal tube above the anastomosis.
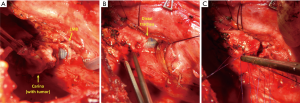
To complete the procedure, a systematic hilar and mediastinal lymph node dissection is performed and a pedicled tissue flap is routinely wrapped around the anastomosis. At the end of the procedure, the anastomosis is checked using bronchoscopy, and any secretions are removed from the airways. Patients are usually extubated in the operating room.
Adjuvant therapy
Postoperative chemotherapy or radiotherapy was given under the care of the referring physicians. No standard protocol was defined. Twenty-nine patients had adjuvant therapy (radiotherapy in 18 patients, chemo-radiotherapy in 6, chemotherapy in 4, and brachytherapy in 1).
Statistical analysis
Survival was estimated from the date of surgery to death or last follow-up. Perioperative mortality included all deaths occurring within 30 days from the operation or during the same hospitalization, if longer than 30 days. Survival probabilities were calculated using the Kaplan-Meier method. Statistical differences between the individual curves were assessed with the log rank test. A value of p less than 0.05 was considered to indicate statistical significance.
Results
Pathology report
Table 1 resumes the main pathologic findings of the study cohort. Final histological examination revealed squamous cell carcinoma in 43 patients, adenocarcinoma in 10, ACC in 7, large-cell carcinoma in 1 and small-cell lung cancer in 1. This patient had a preoperative diagnosis of squamous cell carcinoma. Finally, in one case of a patient with a squamous cell carcinoma who underwent preoperative radiotherapy, no viable tumour cells were found in the resected specimen. The carina or tracheobronchial angle was involved in 33 cases (T4 tumours), while in 29 patients, the tumour involved the origin of the main bronchus (T3) but standard pneumonectomy was not technically feasible. Radical resection was achieved in 52 (82.5%) cases; in 11 (17.5%) cases, microscopic residual disease was detected at the final histological examination. Generally, R1 resections margins were seen at the bronchial or tracheal ends when the maximal length of the airway had already been removed, this was frequently observed in ACC with perineural invasion (n=5). In 4 cases, positive resection margins were described at the final pathology report despite a negative result of the frozen section examination. Nodal status was N0, N1, N2 and N3 in 22 (34.9%), 22 (34.9%), 18 (28.6%) and 1 (1.6%) cases, respectively. Final pathologic stage, according to the 8th edition of the TNM staging system, was stage IIB (12 T3N0) in 12 patients, stage IIIA (8 T3N1, 9 T4N0, 14 T4N1) in 31, stage IIIB (5 T3N2, 13 T4N2) in 18, and stage IIIC (T4N3) in one.
Postoperative morbidity and mortality
The overall perioperative mortality rate was 9.5% (n=6). Causes of death included respiratory failure (n=3), heart failure (n=1), anastomotic fistula (n=1) and pulmonary embolism (n=1). Two of these patients received induction chemotherapy.
Overall, postoperative complications occurred in 26 patients (41.3%), they consisted of cardiac arrythmias in 10 patients, pulmonary oedema in 6, anastomotic complications in 3, empyema in 2, haemothorax in 2, sputum retention in 2 and other complications in 4 (paralytic ileus, chylothorax, wound site infection and acute pulmonary thromboembolism).
Late empyema (arising after 60 days from surgery) without bronchial fistula developed in 6 patients, one of them had an oesophageal fistula.
Survival and follow-up
After a median follow-up time of 131 months (range, 14–290 months), 8 patients (14%) are alive and disease-free. Other 2 subjects had a local relapse, which was treated by surgery in 1 case, and radiotherapy in another case, and they are currently alive without evidence of disease. Of the remaining 47 patients, 22 died of distant metastases, 7 died of a local recurrence, while the other 18 patients died of non-cancer-related causes.
The 2-, 5- and 10-year survival rates were 52%, 31.6% and 20.9%, respectively (Figure 2). Table 2 shows the univariable analysis of most important risk factors for survival. According to nodal stage, the 5-year survival rate was 57.4% for N0 patients, 26.0% for N1 and 7% for N2 patients (P<0.001). Survival related to histology was better for ACC, than for squamous cell or adenocarcinoma (5-year OS rate of 85.7%, 27.3% and 20%, respectively; P=0.035). Positivity of resection margins and T4 status (compared to T3) had no significant impact on survival (P=0.10 and P=0.30, respectively).
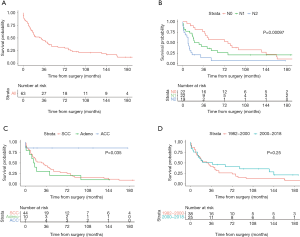
Table 2
| Variable | Number [%] | 5-year survival, % | P |
|---|---|---|---|
| Histology | 0.035 | ||
| Squamous cell | 44 [70] | 27.30 | |
| Adenocarcinoma | 10 [16] | 20.00 | |
| ACC | 7 [11] | 85.70 | |
| Resection margins | 0.1 | ||
| R0 | 52 [83] | 29.80 | |
| R1 | 11 [17] | 39.00 | |
| T status | 0.3 | ||
| T3 | 29 [46] | 37.90 | |
| T4 | 33 [52] | 23.70 | |
| N status | <0.001 | ||
| N0 | 22 [35 | 57.40 | |
| N1 | 22 [35] | 26.00 | |
| N2 | 18 [29] | 7.00 | |
| Date of operation | 0.25 | ||
| 1982 to 2000 | 38 [60] | 26.30 | |
| 2000 to 2018 | 25 [40] | 41.10 |
Thirty-eight patients (60%) underwent TSP from January 1982 to December 1999, while 25 of them were operated after January 2000. The 2- and 5-year survival rate was 52.6% and 26.3%, respectively, in the first group and 50.8% and 41.1% in the latter (P=0.25) (Figure 2).
Discussion
Indications for TSP are unusual, and even the most experienced centres perform only few of such procedures per year (7-9). We noted a modest reduction in the number of cases performed in later years (40% of cases were performed from 2000 to 2018). This could be due to a more accurate patients’ selection, which was certainly favoured, from year 2000, by the addition of PET/CT scan to preoperative workup. Moreover, recently, we described alternative techniques of right extended pneumonectomy for patients with involvement of the tracheobronchial angle that avoid the need to completely remove the carina (10). This may have contributed to the overall reduction of patients undergoing TSP.
We noted a relative increase in the number of patients affected by rarer tumours of the airway, such as ACC. This represents a very different indication from NSCLC, since no established chemotherapy protocols are available in this case, and therefore, surgery represents the mainstay of therapy. Nevertheless, ACC is considered a relatively low-grade malignancy, with a more indolent course and a less frequent involvement of mediastinal lymph nodes than NSCLC. In our study, in fact, survival by histology was significantly better for patients affected by ACC compared to either squamous cell carcinoma or adenocarcinoma (P=0.035).
The main surgical principles of TSP remained essentially the same as those proposed in our previous work (4); however, over the years, we introduced some minor modifications in our operative routine. Firstly, we varied our intraoperative ventilation management strategy, as we found that cross-field intubation with intermittent apnoea, or ventilation with a single-lumen extra-long endotracheal tube are valid alternatives to HFJV. We don’t have arguments to prefer any ventilation modality over the other. In fact, despite early concerns that the use of HFJV could result in increased rates of ARDS (11), later case-series have confuted these findings (4,8). Secondary, in our earlier report, coverage of the anastomosis with a vascularized tissue flap was reserved only for patients at higher risk of dehiscence (longer airway resection, extensive lymph node dissection, or induction therapy). We now apply this precaution in all cases, due to our feeling that this measure is effective in reducing the risk of airway dehiscence. In a similar clinical scenario, in fact, we have observed a significant reduction in the rate of postpneumonectomy bronchopleural fistulae when we started to routinely cover the bronchial stump with pedicled tissue flaps (12). Lastly, supported by our growing confidence in video-assisted thoracoscopic surgery (VATS), we incorporated this type of surgery into a hybrid approach to left TSP, consisting in a right thoracotomy and left VATS. A similar surgical strategy has already been described by other authors (13,14), and we believe that it is extremely useful to deal with particular, more complex cases of left TSP.
Overall, long-term results are in line with those of published literature (1,8,9), with a 5-year OS rate of 31.6%. N2 status remains the most important prognostic factor for survival (P<0.001).
The seemingly high rate of N2-positive patients in our series (28.6%), despite a thorough preoperative staging, might be justified by the anatomical proximity of lower paratracheal (4R and RL) and subcarinal (7) lymph node stations to tumours that involve the carina or the tracheobronchial angle. In this context, it is possible to presume that positive lymph nodes at this level might result from local extension of the tumour, rather than from diffusion through the lymphatic system. Other authors have proposed a treatment algorithm for tumours with carinal or tracheobronchial angle involvement that is based on the type of N2 stations involved; patients with positive ipsilateral lower lymph node stations are selected to undergo induction chemotherapy and surgery, while more distant lymph node spread (e.g., 2R or 2L) represents an absolute contraindication to surgical treatment (15).
Finally, despite our growing experience, and the ameliorations in multimodality therapies and in overall medical care in later years, there was no significant difference in survival between patients operated from 1982 to 2000 and those operated between 2000 and 2018. Certainly, TSP is an important challenge for the thoracic surgeon as morbidity and mortality rates associated with this procedure remain elevated. Therefore, it is of uttermost importance that patients under evaluation for TSP be referred to experienced centres, and that treating physicians have a high index of suspicion for any complication that might arise in the postoperative period. The key to achieving good outcomes is to pay attention to a lot of minor details, from patient selection, to surgical technique and, finally, to postoperative care.
Limitations of this study include its retrospective design and the relatively low number of cases. Because of the low number of events, multivariable analysis of risk factors for survival was not performed; therefore, there is potential for substantial interaction between the analysed independent variables. Nevertheless, the number of included patients compares favourably with that of other published case-series (1,8,9); moreover, the finding of a poor prognostic value associated with a pathologic N2 status is consistent across studies (8,9).
Conclusions
To conclude, TSP is a challenging procedure, associated with high rates of morbidity and mortality. Nodal status is the single most important predictor of long-term survival and, therefore, accurate mediastinal staging is fundamental for patients’ selection. Meticulous operative technique and attentive postoperative management are the foundation for good surgical results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.10.03). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Vergani C, Maciocco M, et al. Tracheal sleeve pneumonectomy: long-term outcome. Lung Cancer 2006;52:105-10. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. J Thorac Dis 2016;8:S882-8. [Crossref] [PubMed]
- Orlowski TM, Dziedzic D. Carinal Resection and Reconstruction. Thorac Surg Clin 2018;28:305-13. [Crossref] [PubMed]
- Rea F, Marulli G, Schiavon M, et al. Tracheal sleeve pneumonectomy for non small cell lung cancer (NSCLC): short and long-term results in a single institution. Lung Cancer 2008;61:202-8. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Grillo HC. Carinal reconstruction. Ann Thorac Surg 1982;34:356-73. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Techniques of pneumonectomy. Sleeve pneumonectomy. Chest Surg Clin N Am 1999;9:407-17. [PubMed]
- Regnard JF, Perrotin C, Giovannetti R, et al. Resection for tumours with carinal involvement: technical aspects, results, and prognostic factors. Ann Thorac Surg 2005;80:1841-6. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg 2001;121:465-71. [Crossref] [PubMed]
- Schiavon M, Marulli G, Rebusso A, et al. Techniques of right extended pneumonectomy. Ann Thorac Surg 2013;96:2252-5. [Crossref] [PubMed]
- Porhanov VA, Poliakov IS, Selvaschuk AP, et al. Indications and results of sleeve carinal resection. Eur J Cardiothorac Surg 2002;22:685-94. [Crossref] [PubMed]
- Mammana M, Marulli G, Zuin A, et al. Postpneumonectomy bronchopleural fistula: analysis of risk factors and the role of bronchial stump coverage. Surg Today 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Ai B, Liao Y, Zhang Z, et al. Single-stage bilateral thoracic surgery via a combined VATS and open approach for left central bronchogenic carcinoma with carinal invasion: report of two cases. J Cardiothorac Surg 2015;10:76. [Crossref] [PubMed]
- Aim A, Almre I, Vanakesa T. Combined approach using uniportal video-assisted thoracoscopic surgery in left tracheal sleeve pneumonectomy. J Thorac Dis 2018;10:E584-6. [Crossref] [PubMed]
- Galetta D, Spaggiari L. Early and long-term results of tracheal sleeve pneumonectomy for lung cancer after induction therapy. Ann Thorac Surg 2018;105:1017-23. [Crossref] [PubMed]
Cite this article as: Mammana M, Ferrigno P, Schiavon M, Rea F. Carinal pneumonectomy: a 36-year experience. Shanghai Chest 2020;4:9.


