
Completion pneumonectomy—indications, practical problems and open questions
Indications for completion pneumonectomy (CP)
There are three groups of indications for CP: (I) CP after previous surgery for lung cancer (LC); (II) CP after previous surgery for multidrug-resistant (MDR) and extensively-resistant (XDR) tuberculosis (TB); (III) CP as the treatment option for complications of previous surgery. Each of these indication groups has a completely different rationale, the evidence about some aspects within each indication group is still scarce.
LC (local recurrence, new primary)
CP is reported in up to 4% of patients with loco-regional recurrence or new primaries (1). In order to correctly understand the role of CP in patients with LC, some uncertainties appearing throughout the literature, mostly related to definition and differences in the reported incidence, should be clarified.
In some studies, postoperative local recurrence relates only to the region of the bronchial stump, ipsilateral hilum and mediastinum (2). The other reports extend this definition to ipsilateral lung and contralateral N1 and N2 nodes as well (3). In some reports, there is no clear definition. These inconsistencies affect not only the reported incidence of local recurrence, but the reported proportion of CP as a treatment option as well. Some of the possible explanations can be obtained from the analysis of seven studies of resectable LC, each including more than 300 patients, most of them appropriately addressing the initial cancer stage (4-10). The wide range of the incidence of the local recurrence in these studies (8–24%) can be explained by the applied methodology: in the study reporting only 8% of initial local recurrence, patients with simultaneous locoregional and distant recurrences were reported as distant recurrences only. Furthermore, in additional two of these studies, only patients with stage I were included, causing the lowest local recurrence rate among these seven studies. That is why the true local recurrence rate seems to be closer to 13–24%, as reported in the remaining studies without the described methodological bias.
Surgical considerations
This type of surgery is indicated in case of LC recurrence or new primary after previous surgery for LC or other, non-cancer related pathology, mostly infections (TB, bronchiectasis, abscess), frequently years before the planned redo surgery. This operation may be indicated either after lobectomy/bilobectomy, or even after sublobar resection, depending on the recurrence localization.
Survival
According to data from 10 studies with a sufficient sample size (2,11-19), 5-year survival was under 30% in four studies, in additional three studies it was 30–40%, whilst in three studies it was over 40% (44%, 44.5%, and 57%). Studies reporting survival worse than 25% included a quite high number of patients with stage III (up to 66%).
As expected, the evidence shows a clear trend of correlation between local recurrence and increasing initial cancer stage, mostly due to the greater likelihood of lympho-vascular invasion (7). However, one of the most challenging findings in this area arise from the studies demonstrating that survival after recurrence may be independent of the stage of the original cancer, but depends rather on the location of the recurrence and of the post-recurrence treatment (20). These findings should not be so surprising, because stage I primary tumors, still carry a 4–19% risk of developing local recurrences after surgery (8), (21,22). The usual explanation is understaging, that occurs in around one-third of stage I patients, mostly because of the difficulty to detect lymph node infiltration by the tumour (23). Understaging may lead to suboptimal extent of the resection, increased recurrence rate and subsequent need for CP.
In a multi centric study on 165 patients, the 5-year survival for squamous and adenocarcinoma was 48.9% and 23.9%, respectively (24). Although a trend of a worse prognosis of recurrent adenocarcinoma vs. squamous cell carcinoma was reported, this aspect is still not sufficiently evidence based.
Concerning prognostic significance of the interval between the two surgeries, according to rare studies with more than 50 patients, it seems that survival is better if the interval is longer, but again without statistical significance (35.4% vs. 54.6% 5-year survival for intervals <2 and >2 years, respectively) (1). Some opposite trends come from studies on smaller patient groups (25).
Related to survival differences between LC recurrence and new primaries, the literature data vary between no survival differences (38% vs. 41%) (26), through slightly (44.6% vs. 29.2%) (1) to significantly better survival for second primaries, with only 10% of the patients with a recurrent carcinoma surviving 21 months (19). The same survival trend was confirmed by some other studies (12). The cause of such a difference in the reported data is inconsistent inclusion criteria.
OpMb, OpMt
The main drawback that has traditionally been attributed to CP is the high operative morbidity. However, as operative mortality was rarely reported to exceed 12%, with operative morbidity being <40%, these complication rates seem to be comparable with those after standard pneumonectomy (27).
The reported rate of bronchopleural fistula (BPF) is 7.0–13.3% in the majority of the series. Even in the biggest series, either no significant risk factors were identified, or some of them were identified only by univariate analysis, like for example, predicted-postoperative (ppo) forced expiratory volume in 1 second (FEV1) <50% (24). Many reports, including our experience, are strongly in favor of bronchial stump protection, especially for right-sided operations. The current evidence is not big enough to reliably recommend manual vs. stapler closure of the bronchus. Currently, mechanical staplers seem to be the preferred technique by most surgeons, depending on countries and technical facilities.
One of the risks that are not to be neglected in patients undergoing a CP is the risk of intraoperative death, occurring in around 5.3%. Injury of the great vessels or heart, usually due to major pleural or pericardial adhesions after the first surgery are reported as the main causes of death (28). To avoid such incidents, we advocate early opening of the pericardium, rather than dissection around shortened vessels surrounded by thickened tissue.
Preoperative work up
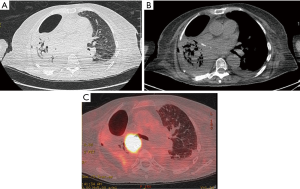
Apart from the adherence to the well-established criteria for functional operability and meticulous high-resolution CT and positron emission tomography (PET)-CT analysis, endoscopic assessment of the local disease spread is the key-point for completeness of the resection and complications prevention. One should be aware of a possible submucosal disease spread proximal to the level of the visible endobronchial tumour. The main bronchus may be invaded at the level of the planned transection, or even proximal. In borderline cases, a bronchial biopsy from these sites is mandatory. Extraluminal compression to the bronchus near the planned level of the bronchus cut, should be also seriously taken into account, even in presence of the normal mucosa. Although a completion sleeve pneumonectomy may be an alternative for such a scenario, one should be aware that the mobility of the left main bronchus may be reduced due to severe tissue reaction after previous adjuvant or neoadjuvant chemo- or chemo/radiation therapy, thus highly increasing the risk of anastomosis insufficiency. For this reason, a preoperative bronchoscopy should be done either by the operating surgeon or by experienced bronchoscopist with endoscopic photos or video-clips whenever possible. An example of CP after previous chemo-radiation treatment is presented on Figure 1.
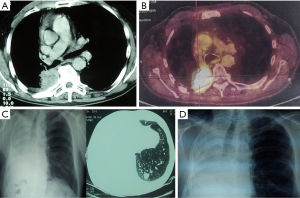
Adjuvant therapy
The patient’s fitness for eventual subsequent adjuvant treatment is very important in this patient group. Quite a high proportion of patients with CP (almost a half of them) can be safely exposed to this kind of treatment (25). Despite favorable results of five largest adjuvant chemotherapy trials with a confirmed survival benefit at 5 years (29-31), it is still unclear whether such a treatment would be appropriate after re-do surgery as well.
Surgical alternatives to CP
CP, it is not the only surgical option for re-do surgery in LC patients. A possible alternative to CP after sublobar resection may be a rest-lobectomy of the recurrent tumour-bearing lobe. Not infrequently, this option requires some of the broncho- or vasculoplastic procedures. Similarly, an alternative to CP after lobectomy may be a wedge/anatomic segmental resection. Segmental resection may be technically more demanding in this situation. For both alternatives, the main problems relate to the postoperative air leak and unclear oncological benefit. The only study specifically addressing the postoperative air leak after redo surgery by comparing the efficacy of Tacho-Seal vs. traditional aerostasis procedures (stapling, suture), revealed some interesting findings outside the primary study endpoints: despite the clear reduction of air leak (4.7 vs. 10.0 days), earlier chest tube removal and shorter hospital stay in favor of the Tacho-Seal group, the frequency of postoperative atrial fibrillation was higher in this group. Similar results were also reported by other authors, explaining it as coincidental finding (32). These reports confirm that major cardiac rhythm impairments may affect patients with air leak in the first postoperative days, despite the use of efficient surgical sealants. Indeed, atrial fibrillation is one of the most common clinical complications in non-cardiac thoracic surgery (33-35), and its frequency is statistically increased in redo surgery. These findings should be taken into account when deciding between CP and possible alternatives, even if technically feasible, especially in patients with preexisting cardiorespiratory comorbidity.
Alternative nonsurgical treatment options
The complexity and some unclear oncological aspects of CP impose the need to seriously consider the possible alternative nonsurgical treatment. Although the combined radio-/chemotherapy is a promising option for patients with a post-surgical recurrence, as shown in the single-centre study of Takenaka et al. (36), studies comparing repeat surgery with radio-/chemotherapy or with radiation therapy alone are missing. Furthermore, favoring alternative treatment by comparing survival of patients with postoperative recurrence with survival of patients with newly diagnosed non-small cell lung cancer (NSCLC) treated with radiation therapy only or with combined radio-/chemotherapy, is methodologically debatable (37). In addition to these considerations, in patients treated by stereotactic radiation therapy (STRT) pathological diagnosis is not obtained and treatment evaluation is difficult because of the inevitable inflammatory response and fibrosis (38,39). That is why radiation treatment currently remains an alternative treatment for functionally inoperable patients with postoperative LC recurrence, or for patients with technically nonresectable tumours.
Disease relapse after surgery for MDR/XDR lung TB
Patients requiring a CP constitute quite a small part of patients undergoing surgery for the lung TB. In these patients, CP represents the only potentially curative option after the failure of previous surgery. Each year an estimated 500,000 MDR-TB cases develop worldwide, only 7% being diagnosed and treated (40). Apart from the former Soviet Union, having the highest rates of MDR- and XDR-TB in the world, reports that appeared after the surveillance study of XDR-TB in 2006, demonstrated high rates in the WHO European Region as well (41).
Rationale for CP in patients with TB
A rationale behind the need for CP for TB is a quite limited treatment success rate for MDR- and XDR-TB. Based on the analysis of 33 cohort studies with over 8,000 cases of MDR-TB, an overall treatment success rate was 62% (42). As for XDR-TB, a 38–57% success rate was reported in human immunodeficiency virus (HIV)-negative persons, 1-year mortality being 81–98% in HIV-positive patients (43-45). Furthermore, redo surgery may be indicated not only for disease caused by M tuberculosis, but also for those caused by environmental mycobacteria (EM). These include M avium complex, M xenopi, and Mycobacterium kansasii, as well as M abscessus, Mycobacterium fortuitum, and M chelonae. The incidence of this type of infection is increasing (46).
Reports that preoperatively sputum-negative patients often have positive cultures in resected lung tissue (27–100%), additionally explain the need for subsequent redo surgery (47,48).
Based on 18 case series studies, including 964 drug-resistant TB patients (895 MDR/69 XDR) (49), reoperation rates for persistent or recurrent TB were low, with the exception of one study in which 11% required reoperation for persistent disease (50).
Reports about CP as a type of surgery vary between not analysing it separately from the entire number of pneumonectomies, through presenting it as percentage of performed pneumonectomies, like 2/59 (3.4%) (51) or 4/94 (4.3%) (52) to reports dealing with CP either as the only analysed cohort (53) or as a substantial proportion (37.4%) of pneumonectomies like in a French national survey (54). Despite these limitations, several lessons have been learned.
Indications for CP
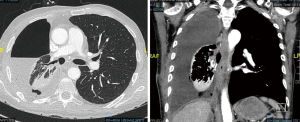
Indications for CP in patients with MDR/XDR-TB are similar to those for primary resectional surgery. Redo surgery is needed for chronic, persistent disease, with failure to thrive, persistent localized cavitary disease or destroyed lung, persistent sputum positivity, hemoptysis, BPF, and bronchial stenosis. An example of CP after previous lobectomy is presented on Figure 2.
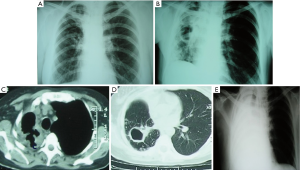
As for the patients with EM, due to the indolent disease course, many of them become severely debilitated. Most of these patients are unable to work, posing significant socio-economic burdens. The analysis of 26 CP for chronic MB disease supports such a statement—77% of the patients were below their ideal body weight, and 77% had anemia as well. Despite aggressive nutritional supplementation, the average preoperative albumin level could not be higher than 3.1 g/dL (53).
Preoperative work up
Before offering a CP as a treatment option, one should check whether the patient underwent an appropriate chemotherapy after the initial surgery. According to current recommendations, for culture-positive patients at time of initial surgery, 4–6 months of therapy after culture conversion is recommended, provided TB was drug-susceptible; for patients with MDR-TB at least 18 months of therapy after culture conversion, whilst in case of XDR-TB, at least 24 months of therapy is recommended after the culture conversion. For patients that were culture-negative at the time of surgery, at least four months of therapy after surgery if TB was drug-susceptible and 6–8 months of therapy after surgery for MDR/XDR-TB (55). Of course, in many patients requiring a CP, it is not possible to fulfill these recommendations because of rapidly progressive disease and patient deterioration. However, even in this situation, absence of progressive disease in the contralateral lung is mandatory.
Unlike a general consensus about TB drug therapy, data regarding optimal drug treatment for EM are still lacking, making surgical decisions more difficult. The usual duration of therapy is 2 years, because of the reported increased risk of relapse after shorter regimens (56).
Before subjecting a patient to CP, a bronchoscopic biopsy of bronchial mucosa, aimed to exclude TB, is mandatory (57). The existence of TB at the level of bronchial mucosa is a contraindication for surgery, as it may predispose to the development of BPF (58). Bronchial stenosis, often combined with a TB destroyed lung, should be clearly distinguished from TB of the bronchus (59); however, it does not constitute an obstacle for pneumonectomy, as presented on Figure 3.
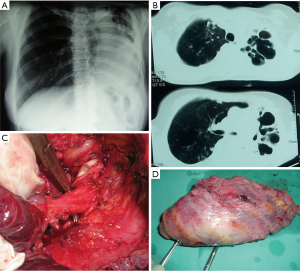
Operative technique
CP for TB may be technically even more difficult than CP for malignant disease. The main problems relate to difficulties in peribronchial and intrapericardial dissection due to adhesions between the pericardium and blood vessels. Related to the bronchial closure technique, the practice varies widely, no firm evidence based recommendations can be given, with the exception of the broad consensus about the need of bronchial stump coverage. There are also reports about the change of the technique with accumulating experience, like switching from stapled bronchial closure, to a tailored suture closure (53).
Survival
Rare papers report on long-term survival after pneumonectomy for TB, including a CP, but without a separate analysis. In the study of Ashour, OpMt was zero after 12–124 months of follow-up of 24 patients with pneumonectomy for TB (60). In a group of patients with preoperative empyema, Shiraishi reported a 5-year survival of 83% during up to18.8-year follow-up (61). In one additional study by Kim et al. 5- and 10-year survival rates were 94%±3% and 88%±4%, respectively (52).
Among the 17 long-term survivors in the aforementioned series of Sherwood et al. (53), sputum conversion or discontinuation of medications was achieved in 14 (82%).
OpMb, OpMt
Pneumonectomy for inflammatory lung disease, especially lung TB, is a high-risk procedure and several authors recommend it to be avoided whenever possible (62). Wide range of complication rate is reported depending on patient group characteristics, ranging from 23% to 63% morbidity and 0% to 25% mortality. In the French national study, dealing with a high number of CP for TB, OpMt was also high (22.15%), with OpMb being higher for CP vs. standard pneumonectomy: 62.5% vs. 47.3% (P=0.008) (54). Such a high mortality was attributed to emergency operations in this series, being the only independent prognostic factor of operative mortality.
The 4.0–8.5% operative mortality of pneumonectomy for inflammatory lung disease seems to mirror the situations in most of the centers.
Despite a discrepancy regarding operative mortality, most of the authors agree that morbidity remains high, postpneumonectomy empyema being the major problem. The reported incidence of empyema ranges from 5% to 32% (63,64).
In a biggest series with CP for EMB, operative mortality and morbidity were 23% and 46%, respectively (53).
Complications of previous surgery
In addition to the sufficiently evidence based indication for CP in patients with persistent air leak or BPF after previous lung resection, here we focus to the role of CP in two additional situations: (I) lung torquation after previous lung resection; (II) CP after previous pleural empyema.
Lung torquation after previous lung resection
The reported incidence of the lung torquation is 0.2–0.3% after thoracotomies (65). CP as a treatment option is more frequently considered after left-sided lobectomies, because 85% of all postoperative torquations were reported after the right upper lobectomy (66), usually with middle lobe torquation and middle lobectomy as a valuable option, without the need for CP.
The evidence about this topic is scarce, in form of case reports or rare case series. The only systematic review of this topic, that included 109 patients from 91 studies showed that presenting symptoms, either acute or indolent, are not typical and the diagnosis is suspected based on radiological findings (67). If the progressive lung consolidation is identified on the chest X-ray, thoracic CT and/or bronchoscopy is the next step. If CT shows bronchial or vascular malposition or if bronchoscopy shows narrow or distorted bronchus orifice, lung torquation is highly suspected.
Differential diagnosis includes pneumonia and lung gangrene. As both diseases are characterized by arterial or venous obstruction, lung gangrene may mimic the lung torquation.
Concerning treatment, two options are available—detorquation (repositioning) of the affected lobe or lung and the lung resection. Major arguments against reposition are embolism from the obstructed pulmonary vein (68) and releasing of inflammatory mediators from the affected lung segment (69), leading to acute respiratory distress syndrome (ARDS) and possibly multiorgan dysfunction. However, several reports of successful reposition suggest that the preserved arterial flow is the key-factor (70,71). It can be assessed by intraoperative ultrasound, if available. Some authors suggest that detorquation may be considered only if the torquation is recognized the first day of onset (72).
One should not hesitate with CP (or lobectomy on the right side, if possible) if the affected lung appears as swollen, airless, with blue-black hemorrhagic surface. Resection may be direct (without detorsion) or indirect (resection with detorsion). Indirect resection is technically easier, but severe reported complications like ARDS, and an extensive systemic circulation embolism should not be neglected. Some authors suggest (73) a direct resection to avoid a reperfusion insult. An example of indirect resection is presented on Figure 4.
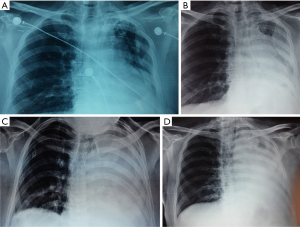
CP after previous pleural empyema
The need for CP after previous empyema represents a major surgical challenge. The postoperative pleural empyema is more frequent than pleural empyema complicating the natural course of LC, being 0.1% to 0.3% (74).
Surgical treatment of LC after previous pleural empyema has been rarely reported (75,76). In a series on 12 pneumonectomies after empyema, OpMb was 33% with no mortality (77). There are two groups of indications for CP after pleural empyema: (I) new or recurrent LC after previous lung resection that was subsequently complicated by empyema, as presented on Figure 5; (II) in patients in whom it is not possible to achieve the lung expansion after lobectomy, independently of indication, as presented on Figure 6.
In patients with the first group of indications, the surgical strategy may be twofold: the first comprises conservative treatment of empyema, usually by prolonged chest tube aspiration (or, rarely, repeated thoracocenthesis), followed by CP after healing of empyema and pleural space obliteration. The second approach includes upfront surgery with intraoperative and postoperative repeated (in 3–4 days intervals) tamponade of the postpneumonectomy cavity with povidone iodine gauzes, or by performing repeated tamponade with specially designed vacuum-system (VAC).
In patients with the second group of indications, the second approach is the only available option, given the impossibility of the lung expansion.
The key-point of the first surgical approach is whether and how long it may be justified to postpone surgery until full control of infection and empyema. According to rare reports, this period was 2 weeks to 1 month, rarely longer. The evidence is not strong enough to answer this question. We incline to believe that such an approach may be justified in presence of the true putrid empyema and septic condition of the patient, obviating the possibility of upfront surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.09.04). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guggino G, Doddoli C, Barlesi F, et al. Completion pneumonectomy in cancer patients: experience with 55 cases. Eur J Cardiothorac Surg 2004;25:449-55. [Crossref] [PubMed]
- Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg Oncol 2013;22:156-61. [Crossref] [PubMed]
- Varlotto JM, Recht A, Flickinger JC, et al. Varying recurrence rates and risk factors associated with different definitions of local recurrence in patients with surgically resected, stage I nonsmall cell lung cancer. Cancer 2010;116:2390-400. [PubMed]
- Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg 2012;93:1813-20. [Crossref] [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 2009;115:5218-27. [Crossref] [PubMed]
- Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007;83:409-17; discussion 417-8. [Crossref] [PubMed]
- Saynak M, Veeramachaneni NK, Hubbs JL, et al. Local failure after complete resection of N0–1 non-small cell lung cancer. Lung Cancer 2011;71:156-65. [Crossref] [PubMed]
- Nakagawa T, Okumura N, Ohata K, et al. Post-recurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:499-504. [Crossref] [PubMed]
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192-6. [Crossref] [PubMed]
- McGovern EM, Trastek VF, Pairolero PC, et al. Completion pneumonectomy: indications, complications, and results. Ann Thorac Surg 1988;46:141-6. [Crossref] [PubMed]
- Grégoire J, Deslauriers J, Guojin L, et al. Indications, risks and results of completion pneumonectomy. J Thorac Cardiovasc Surg 1993;105:918-24. [PubMed]
- al-Kattan K, Goldstraw P. Completion pneumonectomy: indications and outcome. J Thorac Cardiovasc Surg 1995;110:1125-9. [Crossref] [PubMed]
- Verhagen AF, Lacquet LK. Completion pneumonectomy: a retrospective analysis of indications and results. Eur J Cardiothorac Surg 1996;10:238-41. [Crossref] [PubMed]
- Muysoms FE, de la Riviere AB, Defauw JJ, et al. Completion pneumonectomy: analysis of operative mortality and survival. Ann Thorac Surg 1998;66:1165-9. [Crossref] [PubMed]
- Tronc F, Gregoire J, Rouleau J, et al. Techniques of pneumonectomy: completion pneumonectomy. Chest Surg Clin N Am 1999;9:393-405. [PubMed]
- Fujimoto T, Zaboura G, Fechner S, et al. Completion pneumonectomy: current indications, complications, and results. J Thorac Cardiovasc Surg 2001;121:484-90. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Falezza G, et al. Completion pneumonectomy for non-small cell lung cancer: experience with 59 cases. Eur J Cardiothorac Surg 2002;22:30-4. [Crossref] [PubMed]
- Jungraithmayr W, Hasse J, Olschewski M, et al. Indications and results of completion pneumonectomy. Eur J Cardiothorac Surg 2004;26:189-96. [Crossref] [PubMed]
- Endo C, Sakurada A, Notsuda H, et al. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg 2012;93:1061-8. [Crossref] [PubMed]
- Pisters KMW, Le Chevalier T. Adjuvant chemotherapy in completely resected non-small-cell lung cancer. J Clin Oncol 2005;23:3270-8. [Crossref] [PubMed]
- Choi MS, Park SJ, Kim HK, et al. Analysis of 1,067 cases of video-assisted thoracic surgery lobectomy. Korean J Thorac Cardiovasc Surg 2011;44:169-77. [Crossref] [PubMed]
- Stiles BM, Servais EL, Lee PC, et al. POINT: clinical stage IA non-small cell lung cancer determined by computed tomography and positron emission tomography is frequently not pathologic IA non-small cell lung cancer: the problem of understaging. J Thorac Cardiovasc Surg 2009;137:13-9. [Crossref] [PubMed]
- Cardillo G, Galetta D, Van Schil P, et al. Completion pneumonectomy: a multicentre international study on 165 patients. Eur J Cardiothorac Surg 2012;42:405-9. [Crossref] [PubMed]
- Subotic D, Molins L, Soldatovic I, et al. Completion pneumonectomy: a valuable option for lung cancer recurrence or new primaries. World J Surg Oncol 2018;16:98. [Crossref] [PubMed]
- Chataigner O, Fadel E, Yildizeli B, et al. Factors affecting early and long-term outcome s after completion pneumonectomy. Eur J Cardiothorac Surg 2008;33:837-43. [Crossref] [PubMed]
- Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983;86:654-8. [PubMed]
- Massard G, Lyons G, Wihlm JM, et al. Early and long-term results after completion pneumonectomy. Ann Thorac Surg 1995;59:196-200. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Marta GM, Facciolo F, Ladegaard L, et al. Efficacy and safety of TachoSil versus standard treatment of air leakage after pulmonary lobectomy. Eur J Cardiothorac Surg 2010;38:683-9. [Crossref] [PubMed]
- Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779-86. [Crossref] [PubMed]
- Roselli EE, Murthy SC, Rice TW, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg 2005;130:438-44. [Crossref] [PubMed]
- Onaitis M, D’Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of The Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:368-74. [Crossref] [PubMed]
- Takenaka T, Takenoyama M, Toyozawa R, et al. Concurrent chemoradiotherapy for patients with postoperative recurrence of surgically resected non-small-cell lung cancer. Clin Lung Cancer 2015;16:51-6. [Crossref] [PubMed]
- Cai XW, Xu LY, Wang L, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1100-5. [Crossref] [PubMed]
- Van Schil PE, Van Meerbeeck J. Surgery or radiotherapy for early-stage lung cancer--a potential comparison bias. Lancet Oncol 2013;14:e390 [Crossref] [PubMed]
- Van Schil PE. Results of surgery for lung cancer compared with radiotherapy: do we speak the same language. J Thorac Oncol 2013;8:129-30. [Crossref] [PubMed]
- Caws M, Ha DT. Scale-up of diagnostics for multidrug resistant tuberculosis. Lancet Infect Dis 2010;10:656-8. [Crossref] [PubMed]
- Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J 2009;33:871-81. [Crossref] [PubMed]
- Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009;9:153-61. [Crossref] [PubMed]
- Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010;181:80-6. [Crossref] [PubMed]
- Eker B, Ortmann J, Migliori GB, et al. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerg Infect Dis 2008;14:1700-6. [Crossref] [PubMed]
- Leimane V, Dravniece G, Riekstina V, et al. Treatment outcome of multidrug/extensively drug-resistant tuberculosis in Latvia, 2000–2004. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology 2010;36:584-93.
- O’Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis 1987;135:1007-14. [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Aggressive surgical treatment of multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2009;138:1180-4. [Crossref] [PubMed]
- Park SK, Kim JH, Kang H, et al. Pulmonary resection combined with isoniazid- and rifampin-based drug therapy for patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis 2009;13:170-5. [Crossref] [PubMed]
- Kempker RR, Vashakidze S, Solomonia N, et al. Grand round calling the surgeon: the role of surgery in the treatment of drug-resistant tuberculosis. Lancet Infect Dis 2012;12:157-66. [Crossref] [PubMed]
- Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J 2006;28:576-80. [Crossref] [PubMed]
- Yang L, Ding CL, Chang XJ, et al. Analysis of pneumonectomy for benign disease: a single institution retrospective study on 59 patients. Ann Thorac Cardiovasc Surg 2015;21:440-5. [Crossref] [PubMed]
- Kim YT, Kim HK, Sung SW, et al. Long-term outcomes and risk factor analysis after pneumonectomy for active and sequela forms of pulmonary tuberculosis. Eur J Cardiothorac Surg 2003;23:833-9. [Crossref] [PubMed]
- Sherwood JT, Mitchell JD, Pomerantz M. Completion pneumonectomy for chronic mycobacterial disease. J Thorac Cardiovasc Surg 2005;129:1258-65. [Crossref] [PubMed]
- Rivera C, Aramea A, Pricopi C, et al. Pneumonectomy for benign disease: indications and postoperative outcomes, a nationwide study. Eur J Cardiothorac Surg 2015;48:435-40. [Crossref] [PubMed]
- The role of surgery in the treatment of pulmonary TB and multidrug- and extensively drug-resistant TB. Copenhagen: The Regional Office for Europe of the World Health Organization, 2014.
- Iseman MD. Medical management of pulmonary disease caused by Mycobacterium avium complex. Clin Chest Med 2002;23:633-41. [Crossref] [PubMed]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [Crossref] [PubMed]
- Donath J, Khan FA. Tuberculous and posttuberculous bronchopleural fistula. Ten year clinical experience. Chest 1984;86:697-703. [Crossref] [PubMed]
- Williams DJ, York EL, Nobert EJ, et al. Endobronchial tuberculosis presenting as asthma. Chest 1988;93:836-8. [Crossref] [PubMed]
- Ashour M. Pneumonectomy for tuberculosis. Eur J Cardiothorac Surg 1997;12:209-13. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Koyama A, et al. Morbidity and mortality after 94 extrapleural pneumonectomiesfor empyema. Ann Thorac Surg 2000;70:1202-6. [Crossref] [PubMed]
- Massard G, Dabbagh A, Wihlm JM, et al. Pneumonectomy for chronic infection is a high-risk procedure. Ann Thorac Surg 1996;62:1033-7. [Crossref] [PubMed]
- Blyth DF. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg 2000;18:429-34. [Crossref] [PubMed]
- Conlan AA, Lukanich JM, Shutz J, et al. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg 1995;110:1118-24. [Crossref] [PubMed]
- Larsson S, Lepore V, Dernevik L, et al. Torsion of a lung lobe: diagnosis and treatment Thorac Cardiovasc Surg 1988;36:281-3. [Crossref] [PubMed]
- Wagner RB, Nesbitt JC. Pulmonary torsion and gangrene. Chest Surg Clin North Am 1992;2:839-52.
- Dai J, Xie D, Wang H, et al. Predictors of survival in lung torsion: a systematic review and pooled analysis. J Thorac Cardiovasc Surg 2016;152:737-45.e3. [Crossref] [PubMed]
- Apostolakis E, Koletsis EN, Panagopoulos N, et al. Fatal stroke after completion pneumonectomy for torsion of left upper lobe following left lower lobectomy. J Cardiothorac Surg 2006;1:25. [Crossref] [PubMed]
- Kucich VA, Villarreal JR, Schwartz DB. Left upper lobe torsion following lower lobe resection. Early recognition of a rare complication. Chest 1989;95:1146-7. [Crossref] [PubMed]
- Irie M, Okumura N, Nakano J, et al. Spontaneous whole-lung torsion after massive pleural effusion and atelectasis. Ann Thorac Surg 2014;97:329-32. [Crossref] [PubMed]
- Oliveira C, Zamakhshary M, Abdallah MR, et al. Lung torsion after tracheoesophageal fistula repair: a case report and review of literature. J Pediatr Surg 2007;42:E5-9. [Crossref] [PubMed]
- Takada T, Kuriyama A. Lung torsion. Emerg Med J 2015;32:14. [Crossref] [PubMed]
- Banki F, Velmahos GC. Partial pulmonary torsion after thoracotomy without pulmonary resection. J Trauma 2005;59:478-81. [Crossref] [PubMed]
- Sok M, Dragas AZ, Erzen J, et al. Sources of pathogens causing pleuropulmonary infections after lung cancer resection. Eur J Cardiothorac Surg 2002;22:23-7. [Crossref] [PubMed]
- Riquet M, Hubsch JP, Le Pimpec Barthes F, et al. Purulent pleurisy and lung cancer. Non-iatrogenic forms and therapeutic management. Rev Mal Respir 1999;16:817-22. [PubMed]
- Ernst M, Nies C. Thoracoscopic therapy of pleural empyema after pneumonectomy. Chirurg 1999;70:1480-3. [Crossref] [PubMed]
- Subotic D, Petrov D, Gajic M. Lung resection for lung cancer after pleural empyema. Thorac Cardiovasc Surg 2013;61:612-18. [Crossref] [PubMed]
Cite this article as: Subotic D, Lardinois D. Completion pneumonectomy—indications, practical problems and open questions. Shanghai Chest 2019;3:59.


