Robotic subxiphoid thymectomy: techniques, tips, and tricks
Introduction
The lateral intercostal approach is currently the most popular approach for robot-assisted thymectomy. However, difficulties exist in locating the contralateral phrenic nerve through this approach from a single side, and in securing a clear view of the operative field of the cervical region around the superior pole of the thymus. By contrast, bilateral phrenic nerves can be located more easily by robotic subxiphoid thymectomy while easily securing a clear view of the operative field (1,2). In this article, we describe the techniques of robotic subxiphoid thymectomy, and give tips and tricks.
Surgical indications
The surgical approach can be applied to thymectomy/anterior mediastinal tumorectomy for anterior mediastinal tumors (such as thymic tumor and mature teratoma) and extended thymectomy for myasthenia gravis. The da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) allows for suture operation from a natural angle because of its 3D imaging system, ability to remove physiologic tremor, and presence of a forceps joint that mimics the movement of the human hand joint. We have had experience with pericardium reconstruction (3) and innominate venous replacement using a synthetic graft during robotic subxiphoid thymectomy. However, we expect this approach to be applied to more complicated surgeries, such as combined resection of adjacent organs in thymectomy.
Surgical methods
Here, we describe the surgical techniques for robotic subxiphoid thymectomy when using the da Vinci surgical system.
Body posture, port location, and general anesthesia in the subxiphoid approach
Surgery is performed with the patient under general anesthesia. Patients require differential lung ventilation if lung partial resection is performed as a result of potential tumor invasion to the lung or if the tumor is close to the phrenic nerve such that the margin requires checking. Minimum level pressure control ventilation that allows maintenance of patient respiration is chosen as the ventilator setting, and positive end expiratory pressure (PEEP) is not used. Use of PEEP may disrupt the clarity of the operative field by causing excessive lung expansion. Increased partial pressure of carbon dioxide in blood is managed by increasing the number of ventilations. The intravenous line is placed in the right arm or lower limbs by considering the possibility of clamping in case the left brachiocephalic vein becomes damaged.
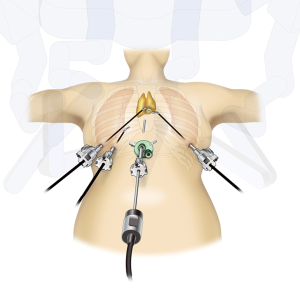
Patients are placed in supine position with the arms and legs open, as the robotic arm may hit the patient’s arm when both arms are placed alongside the body. The legs do not need to be bent. Skin is marked at the following sites: a 3-cm transverse incision site for port incision made 1 cm below the xiphoid process on the caudal side, bilateral 6th intercostal spaces on the anterior axillary line on the lateral side, and right 6th intercostal space on the midaxillary line if the retraction arm is used.
Surgical techniques
The surgeon stands between the patient’s legs to perform the surgical procedure, and the surgical assistant stands on the patient’s right side to operate the camera scope. A 3-cm vertical incision is made approximately 1 cm below the xiphoid process on the caudal side. Hypodermal tissue is resected with an acusector, and the rectus abdominis muscle together with the linea alba are separated from their attachments to the xiphoid process.
The caudal side of the thymus is detached from the sternum blindly using a finger. The linea alba resected from the xiphoid process is felt by the finger as it is moved toward the caudal side. The linea alba is resected further toward the caudal side by approximately 1 cm. A space is then created to allow for port insertion blindly by the finger around the layer at the back of the rectus abdominis muscle. Care must be taken not to damage the peritoneal membrane. If the peritoneal membrane becomes damaged, CO2 will enter the abdominal cavity during insufflation, which will cause abdominal bloating. This may make the surgery slightly trickier; however, it is possible to continue with the surgery. The wound retractor that comes with the GelPOINT Mini® (Applied Medical, Rancho Santa Margarita, CA, USA), which is a port designed for single-port surgery, is inserted through the subxiphoid incision. The GelPOINT Mini® platform is mounted, which has a 10-mm subport for the robot’s camera port insertion and a subport for the surgical assistant. Insufflation and exhaustion tubes are connected to the three-way stopcock of the platform, and CO2 gas is injected at 8 mmHg.
The LigaSure Maryland type (Covidien, Mansfield, MA, USA) is used to detach the thymus from the back of the sternum. There is no problem with using forceps for normal thoracoscopic surgery or hooked forceps instead of Ligasure. As this detachment continues, CO2 injection effectively improves the operative field view by opening up the space at the back of the sternum through exclusion of both the lungs and pericardium.
The bilateral mediastinal pleura is dissected to bilaterally open the chest cavity. The sternum attachment site of the mediastinal pleura is resected to the cervical region. Sufficient space for surgical procedures is secured at the back of the sternum for the following two reasons: (I) bilateral resection of the mediastinal pleura from the sternum causes movement of the pericardium to the dorsal side, and (II) CO2 injection causes exclusion of the pericardium and lungs to the dorsal side. A 1-cm skin incision is made on either side of the 6th intercostal space precordial axillary line, followed by insertion of a robotic surgery port. If a retraction arm is used, the port should be inserted 4–6 cm away from the main port along the anterior to the midaxillary line at the right 6th intercostal space (Figure 1). Generally, two ports are inserted in the right lateral chest. Unlike one’s imagination, the left thoracic wall and the heart are relatively closer when a patient is placed in the supine position. The port for docking the retraction arm is inserted in the right lateral chest to avoid the risk of damaging the heart accidentally by repeated insertion of instruments in the left chest. It is possible to confirm the left and right sides of the thymus sufficiently through traction involving the retraction arm from the right lateral chest. If traction is required from the left lateral chest, the port-hop function can be used to place the camera in the right lateral chest and the retraction arm at the left lateral chest.
In the da Vinci Xi surgical system, the arm is hung from above so that it can be docked from the side. This side-docking allows the anesthesiologist to manage intratracheal intubation without being disturbed by the da Vinci surgical system, thus leading to safer surgery.
Next, the port is connected to the da Vinci arm. Initially, the camera scope is used at 30° “up”. “Up” and “down” will be switched after adjusting the depth of the port and after detaching the thymus from the sternum. The assistant supports the surgeon by inserting forceps from the caudal to the subxiphoid port.
I perform surgical procedures using the fenestrated bipolar forceps with soft coagulation on the left robotic arm, and Maryland bipolar forceps with bipolar cautery or spatulas connected to monopolar cautery on the right robotic arm. When using the Maryland bipolar forceps, the generators VIO 3 (ERBE) auto CUT bipolar power 5.5 and soft COAG bipolar power 6.0 are used. The vessel sealer (Intuitive Surgical) is used to resect narrow blood vessels, such as the thymic vein.
Surgical procedures of thymectomy
The thymus is first detached from the sternum. The location of the bilateral internal thoracic veins is confirmed from the thoracic cavity side. The location of the right internal thoracic vein is often easily identified; however, the left internal thoracic vein may occasionally be difficult to locate. The right side is resected up to where it reaches the internal thoracic vein, and the left side is resected up to 1 cm before the left intrathoracic phrenic nerve on the cranial side. Then, the lower thymus is held by the left fenestrated bipolar forceps to detach the caudal inferior pole of the thymus from the pericardium. The mediastinal pleura is resected 1 cm before the phrenic nerve and joined to the pre-resected pleural incision line on the sternum side. The innominate veins are located where the internal thoracic vein flows in. The location of both internal thoracic veins join is predicted, and the innominate veins are exposed by detaching the fatty tissue from the surface of the thymus by slowly stripping off when this site is reached. When the innominate vein on the left (peripheral) side becomes exposed, cranial fat tissue of the innominate vein and right superior pole of the thymus should be set aside from the upper edge of the innominate vein. When the innominate vein on the right (central) side becomes exposed, fat tissue on the inner side of the right internal thoracic vein and right pole of the thymus is detached from the thyroid, brachiocephalic artery, and trachea while protecting the right brachiocephalic vein. The surgical field view is dramatically improved for the cervical region above the innominate veins by holding the superior pole of the thymus and pulling it to the caudal side. The superior pole of the thymus and fat tissue are then detached from the brachiocephalic artery and trachea while protecting the inferior thyroid vein (this can be resected). When these are detached, the innominate vein is completely exposed by pulling the superior pole of the thymus. The thymic vein that flows into the innominate vein from thymic tissue is resected using the vessel sealer to complete thymectomy/anterior mediastinal tumorectomy. The resected thymus and anterior mediastinal tumor are placed in a bag in the mediastinum and removed through the subxiphoid incision (Figure 2). Surgery is completed by inserting a 20-French drain through this incision.

Tips and points to note during surgery
- The camera should be inserted at 30° “up” initially, and should be switched to 30° “down” when detaching the thymus (after being detached from the sternum) from the pericardium. Sometimes, the camera scope touches the sternum when it is 30° “down” and stops cranial movement. When this occurs, a 0° straight camera scope should be used.
- Occasionally, the patients’ right arm and the retraction arm may come into contact. Care should be taken when operating around the caudal side of the thymus with the retraction arm. Such contact may be avoided by locating the insertion port of the retraction arm toward the medial (anterior) side of the body rather than the lateral side. If such contact seems likely to occur, the retraction arm should not be used for detachment of the thymus on the caudal side.
- When pulling the thymus to the left side with the retraction arm using the Xi system, the #4 arm may be changed to the retraction arm, the camera changed to the #2 arm, and the left and right arms changed to the #1 and #3 arms (port hop function).
- When opening up the view of the cervical region, the view will be improved by holding the superior pole of the thymus and pulling to the caudal side, as this will cause exclusion of the left brachiocephalic vein.
- If the caudal side of the left phrenic nerve is difficult to see, it may be viewed more easily by pulling the fat tissue on the pericardium toward the patient’s left using the retraction arm. When using the Xi system, a camera can be inserted into the port in the left thorax to check its location (port hop function).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giuseppe Marulli) for the series “Robotic Mediastinal Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.05.04). The series “Robotic Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Zhang H, Chen L, Zheng Y, et al. Robot-assisted thymectomy via subxiphoid approach: technical details and early outcomes. J Thorac Dis 2018;10:1677-82. [Crossref] [PubMed]
- Suda T. Subxiphoid Robotic Extended Thymectomy with a Pericardial Patch Closure. CTSNet [published 24 May 2016]. Available online: http://www.ctsnet.org/article/subxiphoid-robotic-extended-thymectomy-pericardial-patch-closure
- Suda T. Robotic subxiphoid thymectomy: techniques, tips, and tricks. Asvide 2019;6:176. Available online: http://www.asvide.com/article/view/32332
Cite this article as: Suda T. Robotic subxiphoid thymectomy: techniques, tips, and tricks. Shanghai Chest 2019;3:32.