Robotic resection of posterior mediastinal lesions: indications, tips and tricks
Introduction
Large reviews about the video-assisted thoracoscopic resection of posterior mediastinal tumors confirm that the VATS approach is feasible and safe, associated with a significant reduction of hospital stay and chest tube duration, as well as a sensible reduction in patient’s morbidity and mortality (1). Despite the clinical advantages of the minimally invasive approach for posterior mediastinal lesions, we have to consider that some of them present anatomical features that make a traditional VATS approach technically challenging or at risk of damaging the surrounding structures (2). In these patients a robotic assisted procedure could allow resection of posterior mediastinal tumors that would otherwise require an open approach, reducing the risk of intraoperative injuries. The robotic technique is able to extend the surgeon ability in tissue dissection, thanks to three-dimensional view and the dexterity due to the use of endowrist (3). As in general surgery for upper GI or for perineal surgery, like prostatectomy or low rectum resection, the robotic surgery is indicated for procedures in the thoracic outlet or the pericardiophrenic angle were the traditional mini-invasive surgery shows technical limits due to the use of straight instruments.
Anatomical considerations
The posterior mediastinum is defined as the space located between the two pleural sacs, bordered superiorly by the thoracic inlet, inferiorly by the diaphragm, anteriorly by the pericardium and posteriorly by the spine, including paravertebral space (4). It contains several structures: descending aorta, esophagus, azygos and hemiazygos vessels, sympathetic chain, vagus and splanchnic nerves, thoracic duct, lymph nodes and adipose tissue. Among the masses that can be found in this compartment, the most common are neurogenic tumors (60%); they arise from the intercostal nerve sheath in the 90% of cases (schwannomas and neurofibromas), but they can also originate from the sympathetic ganglia (neuroblastomas, ganglioneuroblastomas and ganglioneuromas) or the paraganglionic cells (pheochromocytomas and non-chromaffin paragangliomas). Though frequently asymptomatic, some tumors, especially if malignant, can be accompanied by clinical manifestations due to bone erosion or involvement of nerve roots and other surrounding structures, as well as hormones production, such as in pheochromocytomas or neuroblastomas. Sometimes they can develop a spinal canal component (the so called dumbbell tumors) connected to the main mass by a small pedicle passing through the neural exit foramen. Neoplastic masses can also be represented by chordomas, chondrosarcomas, Ewing’s sarcomas, esophageal neoplasms, lymphomas, invasive thymomas or metastases. Besides tumoral masses, posterior mediastinum can contain cystic lesions, vascular or spinal abnormalities (aortic aneurysm, varicose veins, lymphangioma or meningocele) and infectious or inflammatory diseases (paraspinal abscess, mediastinitis, sarcoidosis, lymphoid hyperplasia or pancreatic pseudocysts). Mediastinal foregut cysts are due to abnormalities during the embryogenic development of the primitive foregut; they include bronchogenic cysts, esophageal duplication cysts and neurenteric cysts. Bronchogenic cysts are unilocular, lobulated, fluid-filled lesions, usually detected in the carinal region. Esophageal duplication cysts are typically found in the lower mediastinum; they develop along the esophagus, often intramurally, but they rarely communicate with the esophageal lumen. Neurenteric cysts usually take place in the right hemithorax, above the carina; they communicate with the meninges and are associated with vertebral abnormalities, as well as intestinal duplication, imperforated anus and congenital heart disease.
Preoperative surgical considerations and indications for robotic assisted procedure
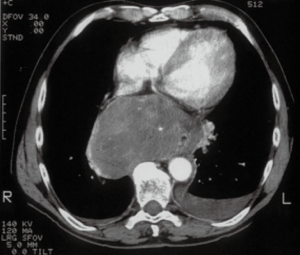
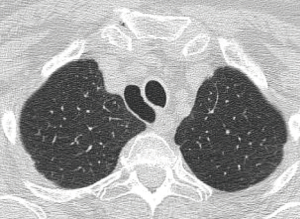
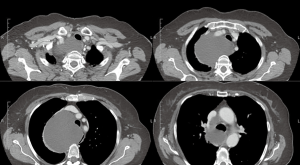
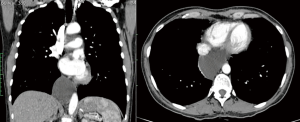
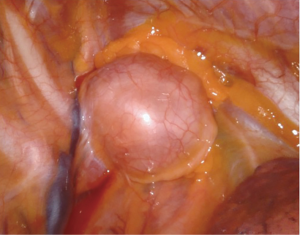
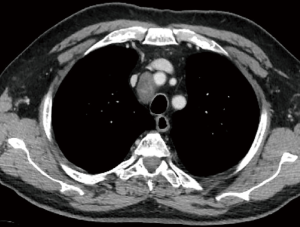
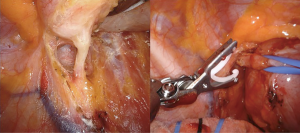
As described above, there are many types of pathologies that could rise in the posterior mediastinum. The first step in the preoperative assessment is to evaluate if the lesion is radically resectable and, in that case, to choose the proper technique (open surgery vs. minimally-invasive procedure). Solid lesions along the costo-vertebral space, especially the ones with a large contact surface, could be cell B lymphomas, in which a simple VATS biopsy is required. On the contrary, in case of giant lesions open surgery must be considered, not only for the problems related to the surgical dissection and to the risk of rupture and seeding, but also for the involvement of surrounding structures like shown in Figure 1, an esophageal gastrointestinal stromal tumor that required a thoracic esophagectomy and gastroplasty. After these preliminary considerations, we could divide the posterior mediastinal lesions in solid, semi-solid or cystic, and cystic with air. The latter ones are the rarest, tracheal diverticula that usually develop in the upper posterior mediastinum and frequently in the thoracic inlet (Figure 2). The precise dissection around the esophagus, with the closure of the small communication with the tracheal lumen at the thoracic inlet, is easier with a robotic assisted procedure compared to traditional VATS (Figure 3). The cystic and semi-solid lesions can rise along the entire posterior mediastinum and they can have a wide range of dimensions; they are benign and a minimally invasive approach is beneficial for the patients. The cystic lesions of the posterior mediastinum can be easily resected with a VATS procedure; however, in case of lesions more than 4 cm located in the upper posterior mediastinum, thoracic inlet or cardio-phrenic angle, close to the esophageal hiatus or inferior cava vein, the robotic approach gives substantial advantages. The first one is the opportunity to make a precise dissection of a large surface reducing surgical time and risk of intraoperative injuries of surrounding structures; the second is the possibility to visualize tiny vascular or lymphatic structures, like in case of mediastinal cystic lymphangiomas (Figure 4), reducing the risk of chylothorax or recurrences as shown in Figure 5. As reported in upper GI, the robotic surgery overcame some technical aspect of esophageal surgery; the same thing can be affirmed in case of big cystic para-esophageal lesions. Figure 6 shows a rare case of branchial cyst close to the lower esophagus, easily resected with robotic technique without any esophageal injury. The solid lesions of posterior mediastinum are usually neurogenic tumors and easy to resect in VATS. However, also in these cases, there are some localizations in which robotic surgery makes the procedure easier and safer. Neurinomas of the first rib (Figure 7) can be resected by a robotic procedure in a fast and precise way; the risk of a Horner syndrome due to a stellate ganglion injury is high and it is mandatory to achieve all the possible precautions to preserve it. Other than the radicular nerves, at that level even the sympathetic chain is often involved (Figure 8) and, for this reason, the use of Harmonic scalpel or advanced bipolar should be recommended (Figure 9). Some neurogenic tumors can rise in the middle mediastinum; also in this case the robotic technique allows the dissection in the retro-caval space and around the trachea, as shown in this case of Schwannoma of the middle mediastinum (Figure 10).



Surgical technique
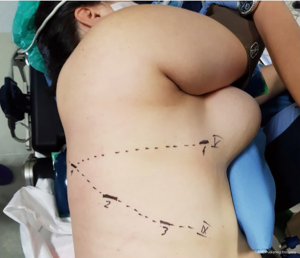
In an effort to standardize the robotic surgical technique for each surgeon, considering the rarity of these lesions, we suggest to insert the robotic trocars along the 9th intercostal space, as reported for robotic lung lobectomy. This disposition enables to explore all the posterior mediastinum from the thoracic inlet to the esophageal hiatus. The use of CO2-inflation, as advocated in some reports, is not necessary in the majority of procedures (8); it is useful only to push down the diaphragm in case of very deep lesions in the posterior costo-phrenic space. For dissection we suggest unipolar hook or the most gently bipolar Maryland forceps. In these cases all the robotic trocars can be positioned along the 9th intercostal space. In our center we prefer to use the robotic harmonic scalpel in the effort to reduce intraoperative bleeding and lymphatic leak or nerves injury. Because the harmonic scalpel is not articulable, it should be positioned in the 5th intercostal space on the right side and in the 9th on the left, maintaining the instrument on the right hand of the surgeon (opposite for left hand). Then, in our technique, three accesses are performed in the 9th intercostal space, approximately 8 cm between each other, starting from the camera port (12 mm) on the middle axillary line and continuing posteriorly for the second and third arm placement. The fourth arm is located in the 5th intercostal space on anterior axillary line, usually equipped with the tip-up forceps for the lung management (Figure 11). The cystic lesions should be dissected from the surrounding tissue trying not to open the capsule and with the effort to close all vascular, lymphatic or airway communications. In case of cysts of the lymphatic duct, usually located in the left side, it is of utmost importance to close the afferent and efferent lymphatic vessels to prevent an important and dangerous postoperative chylothorax; in those cases the use of robotic Hem-o-Lock can improve the lymphatic sealing. In case of suspected oesophageal duplication cysts, an oesophageal endoscopy is recommended to exclude an asymptomatic diverticulum and confirm the separation from the oesophageal lumen.
Conclusions
In case of resectable posterior mediastinal lesions, minimally invasive surgery improve patients outcome in terms of postoperative recovery, pain control and hospital stay. However, the robotic assisted excision of posterior mediastinal tumour is still limited and few cases are reported in literature (9-11). Because of the costs, the robotic surgery should be reserved to patients with lesions that are difficult to resect with traditional VATS procedures or in whom there is a high risk of injury of surrounding structures. The role of robotic surgery should be to offer minimally invasive surgery to patients that would be excluded from a traditional VATS procedure. The possibility to have interchangeable and precise instruments allows dissection in small spaces and close to crucial structures avoiding complications or partial anatomical resection (12,13).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giuseppe Marulli) for the series “Robotic Mediastinal Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.05.03). The series “Robotic Mediastinal Surgery was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hazelrigg SR, Nunchuck SK, LoCicero J 3rd. Video Assisted Thoracic Surgery Study Group data. Ann Thorac Surg 1993;56:1039-43; discussion 1043-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Al-Mufarrej F, Margolis M, Tempesta B, et al. Novel thoracoscopic approach to difficult posterior mediastinal tumors. Gen Thorac Cardiovasc Surg 2010;58:636-9. [Crossref] [PubMed]
- Shields TW, LoCicero J, Ponn RB, et al. General Thoracic Surgery, 6th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2005.
- Luzzi L, De Leonibus L, Ghisalberti M, et al. Robotic resection of a tracheal intra-thoracic diverticulum. Asvide 2019;6:133. Available online: http://www.asvide.com/article/view/31762
- Luzzi L, De Leonibus L, Ghisalberti M, et al. Robotic resection of a cystic lymphangioma. Asvide 2019;6:134. Available online: http://www.asvide.com/article/view/31764
- Luzzi L, De Leonibus L, Ghisalberti M, et al. Robotic resection of a first rib neurinoma. Asvide 2019;6:135. Available online: http://www.asvide.com/article/view/31765
- Nguyen DC, Garagozlo C, Moslemi M, et al. Robotic resection of a superior sulcus neurogenic tumor. Innovations (Phila) 2015;10:142-5. [PubMed]
- Guo W, Yang S, Jin R. Robot-assisted surgery for posterior superior mediastinal mass. AME Med J 2017;2:10. [Crossref]
- Ng CS, Wong RH, Hsin MK, et al. Recent advances in video-assisted thoracoscopic approach to posterior mediastinal tumours. Surgeon 2010;8:280-6. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann Thorac Surg 2002;74:1235-7. [Crossref] [PubMed]
- Pacchiarotti G, Wang MY, Kolcun JPG, et al. Robotic paravertebral schwannoma resection at extreme locations of the thoracic cavity. Neurosurg Focus 2017;42:E17 [Crossref] [PubMed]
- Veronesi G, Solinas M. From manual to robotic video-assisted resection of posterior mediastinal masses. J Thorac Dis 2017;9:2884-7. [Crossref] [PubMed]
Cite this article as: Luzzi L, De Leonibus L, Ghisalberti M, Corzani R, Molinaro F, Ligabue T, Meniconi F, Scolletta S, Paladini P. Robotic resection of posterior mediastinal lesions: indications, tips and tricks. Shanghai Chest 2019;3:27.