
Management options for pulmonary nodules with cancer of unknown primary
Introduction
Cancer of unknown primary (CUP) is defined as a cancer with a histological diagnosis suggestive of a metastasis and incompatible with that of the biopsy site, without identification of a primary site in spite of a comprehensive and exhaustive hunt for the primary including history and examination, blood tests and radiography (1,2).
CUP represents 10% of tumours with metastasis, and between 3-5% of epithelial tumours (3). The use of the full traditional diagnostic armamentarium permits a tissue of origin to be resolved in only about 30% of CUP patients (4). Even post-mortem only identifies the origin of the tumour in about a quarter of cases (5).
Increasingly, histopathology permits anatomical allocation of possible primary sites based on patterns of immunochemical tests (6). This clinic-pathological status permits classification of CUP patients into different outcome groups (7), with differing prognostic histological subsets shown in Table 1; unfortunately only 20% are associated with a favorable prognosis (9).
Table 1
| Favourable subset (20%) |
| 1. Patients with a single small potentially respectable tumor |
| 2. Women |
| • With adenocarcinoma involving axillary lymph nodes |
| • With papillary adenocarcinoma of peritoneal cavity |
| 3. Men with blastic bone metastases and elevated PSA (adenocarcinoma) |
| 4. Poorly differentiated neuroendocrine carcinomas. Merkel cell carcinoma of unknown primary (localized disease) |
| 5. Squamous cell carcinoma involving cervical lymph nodes |
| 6. Adenocarcinoma with a colon-profile (CK20+, CK7, CDX2+) |
| 7. Isolated inguinal lymphadenopathy (squamous carcinoma) |
| Unfavourable subset (80%) |
| 1. Multiple metastases |
| • Cerebral metastases (adeno or squamous carcinoma) |
| • Lung/pleural metastases (adenocarcinoma) |
| • Metastatic bone disease (adenocarcinoma) |
| 2. Poorly differentiated carcinoma |
| 3. Abdomen |
| • Adenocarcinoma metastatic to the liver or other organs |
| • Non-papillary malignant ascites (adenocarcinoma) |
| • Squamous-cell carcinoma of the abdominal cavity |
Thoracic surgeons aim to resect as many lung cancer patients as possible, including marginal patients, thus improving lung cancer resection rates, driven by the belief that that this delivers optimal management and long-term survival rates to patients. However different medical specialities interpret the TNM classification and staging of lung cancer as adapted for the special case of CUP differently, leading to different management choices being offered.
In this perspective, the management and differing perspectives and viewpoints of surgeons, physicians and pathologists in the unusual scenario of patients presenting with a solitary lung mass with histology suggestive of CUP is discussed.
Perspectives
The pulmonologist and radiologist’s perspective
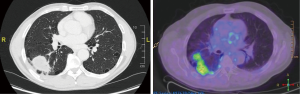
A 61-year-old male smoker was discovered to have an incidental finding of a right lung lesion on pre-operative chest radiography (CXR) prior to right inguinal hernia repair. A CT-scan showed a 5.3 cm by 3.2 cm mass in the apex of the right lower lobe (RLL), a further 6mm nodule in the RLL and a 3 mm nodule in the left upper lobe (LUL), with no lymphadenopathy reported, see Figure 1. A positron emission tomography (PET) scan confirmed the large RLL lesion and showed modest uptake in the right hilum, in keeping with possible hilar lymph node involvement; the nodules were PET negative. To assess hilar involvement, an endobronchial ultrasound (EBUS) was performed, but histology of the biopsy was negative. A CT guided biopsy was then performed; the histological immunohistochemistry indicated adenocarcinoma probably of bowel origin. This was excluded by gastroscopy and colonoscopy, and an MRI abdomen eliminated a pancreatic primary. The patient was referred to Oncology with the presumptive diagnosis of cancer of unknown primary with lung metastasis, making this inoperable disease. Treatment with cycles of Cisplatin and Pemetrexed resulted in a good albeit partial response with diminution in size of the main lung lesion and disappearance of lymph node uptake within the limits of the system’s spatial resolution.
The pathologist’s perspective
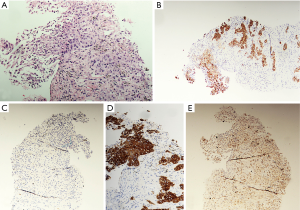
The histology revealed a poorly differentiated carcinoma with glandular differentiation favoring adenocarcinoma (Figure 2). The tumor primarily consisted of solid nests and exhibited an unusual immunohistochemical profile; CK7+, CK20+, TTF1-, CK5/6-, p63+, CDX2-. Given the immunohistochemical panel, a gastrointestinal or bladder primary were considered, with a lung primary less likely.
The surgeon’s perspective
This man was initially referred for a right lower lobectomy with a 5.3 cm RLL mass, with a further small nodule in the same lobe and a tiny nodule in the LUL. However both these two small nodules were PET negative although minimal uptake occurred in the right hilum raising the possibility of early hilar lymph node involvement. Uptake in hilar lymph nodes on PET has a sensitivity of 40% and specificity of 88.7%, with a poor correlation to the final pathologic status of hilar nodes (10). Directed lymph node biopsy using EBUS confirmed clear hilar nodes. Histology of the large RLL lesion had tissue markers suggestive of bowel origin. The pathology was classified as CUP with lung metastases in spite of negative gastroscopy, colonoscopy and PET scan of the abdomen, and the patient was referred to oncology by the pulmonologists, even though the immunochemistry had not ruled out lung primary completely.
From the viewpoint of the surgeon, this case could potentially be classified using lung TNM classification (11) as stage IIB-T3 N0 M0 (>5 cm in greatest dimension, possible separate tumor nodule in the same lobe as the primary, EBUS negative nodes). The counter argument was that this was Stage IV-M1a (separate nodule in contralateral lobe, though PET negative) or CUP with multiple lung metastases. Classifying this as stage IIB would have led to right lower lobectomy, which may have resulted in a potentially curative resection.
This case highlights the ambiguous application of the TNM classification in CUP, with differing interpretations in such cases. It also illustrates the difficulty of managing CUP, especially as the diagnosis hinges on immunohistochemistry that in itself is only indicative of CUP.
Discussion
CUP implies a diagnosis of cancer that has spread but where the primary site remains unknown. It represents 2–3% of all cancers and the location of the primary is never discovered in 40–50% in spite of exhaustive investigation (12). This may be because the primary is very small and slow growing, leaving it outside the definition of modern imaging technology. Another reason may be that the body’s immune system has already destroyed the primary though spread has already occurred through haematological or lymphatic pathways. A third scenario may be that the primary cancer was removed inadvertently through surgical treatment for another condition, for example hysterectomy for menorrhagia or a malignant colonic polyp resected perhaps years before that was subsequently missed during histological examination.
CUP is not a single entity but encompasses a heterogeneous group of cancers. Because the cancer has spread, staging is generally held to be irrelevant and the mainstay treatment is chemotherapy. Patients have by definition advanced disease at the time of diagnosis and therefore non-specific but less effective treatment regimens have to be used that should be effective in all CUP patients (5). This strategy is likely to be conducive to the overall poor prognosis of CUP with a survival of between 2 to 10 months, making CUP the fourth commonest cause of cancer-related death in both genders (13).
Investigation of CUP cases should be thorough in order to verify the presence of metastasis since this affects the decision-making regarding surgery. CT scanning is often the first line tomographic investigation for CUP. CT scan limitations include low detection of small metastasis including distant, peritoneal and non-enlarged lymph node metastasis, with PET permitting scanning of a larger volume than CT.
PET
PET scanning with the use of a glucose analog labeled with radioactive fluorine-18 as a tracer is useful in detecting metastasis. The addition of combined PET/CT for staging results in upstaging disease in 20–30% of patients with NSCLC as compared to conventional staging assessment (14). PET however is limited spatially in the case of tumor size smaller than 4mm due to fundamental limitations in PET machine design (15,16) leading to microscopic disease underestimation. Another limitation is when the tumor has a metabolic profile with low glucose uptake, like bronchoalveolar cell carcinoma and carcinoid tumors (14,17). In spite of these shortcomings, PET scanning has a detection rate for a primary in the context of CUP in 41% cases in a systematic review and meta-analysis of almost three thousand cases (12).
In our case, since the immunohistochemistry of the main lung nodule suggested that the unknown primary was gastrointestinal in origin, PET scanning was especially indicated to exclude pancreatic cancer. As PET scanning is not helpful in T staging stomach cancer (18), gastroscopy was performed, being the modality of choice for diagnosing stomach cancer, with computed tomography (CT) further aiding staging of gastric cancer and its follow-up.
Immunohistochemistry
The combination of morphological features and application of an array of immunohistochemical techniques usually leads to the correct identification of the primary site. Broad-spectrum cytokeratins (pancytokeratins) confirm the epithelial nature and exclude mimics of poorly differentiated carcinomas such as lymphomas, melanomas, and possibly sarcomas. Cytokeratins can be subdivided into high molecular weight cytokeratins (HMWCKs) and low molecular weight cytokeratins (LMWCKs). Adenocarcinomas tend to express LMWCKs such as CK8/CK18 while squamous, transitional, ductal and basal carcinomas express HMWCKs such as 34betaE12. Cytokeratins p63 and CK5/6 are useful in confirming a diagnosis of squamous cell carcinoma (19).
Organ specific cytokeratins
Cytokeratin 7 (CK7) and Cytokeratin 20 (CK20) perhaps form the basis of much of the classification of epithelial tumors. A simplistic rule of thumb is that CK7 positive tumors tend to originate above the diaphragm whilst CK20 positive tumors tend to originate below the diaphragm (20). Furthermore, the co-expression of CK7/CK20 is seen in specific types of tumors; see Table 2.
Table 2
| CK7+/CK20- | CK7-CK20+ | CK7+/CK20+ | CK7-/CK20- |
|---|---|---|---|
| Lung | Colorectal | Transitional cell carcinoma | Hepatocellular |
| Breast | – | Ovarian mucinous | Renal cell carcinoma |
| Ovarian serous | – | – | Prostatic adenocarcinoma |
| Endometrial adenocarcinoma | – | – | – |
There are exceptions to any rule. Stomach, pancreas and biliary primaries can manifest a diverse range of CK7/CK20 pattern of staining; pancreas in particular can exhibit a colorectal immunophenotype. Importantly CK7 and CK20 can stratify CUP into groups, allowing the pathologist to subsequently tailor even more organ specific immunohistochemical stains. Table 3 summarizes some of the more commonly used organ specific markers. There is considerable overlap of immunohistochemical stains and it is beyond the remit of this article to provide an exhaustive list (21).
Table 3
| Organ | Antibodies to |
|---|---|
| Lung adenocarcinoma | Thyroid transcription factor 1 (TTF1), Napsin A |
| Breast | GCDFP-15, GATA3, Estrogen Receptor (ER) |
| Gynaecological | PAX8, Wilms Tumor Antibody (WT1), ER |
| Prostate | Prostate Specific Antigen (PSA) |
| Colorectal | CDX2 |
| Hepatocellular | Hep-Par1 |
| Bladder | GATA3, Uroplakin, p63 |
| Renal | PAX8 |
| Thyroid | TTF1, PAX8, Thyroglobulin |
TNM classification
In the TNM classification for lung cancer, any tumor with separate tumor nodules in the same lobe is categorized as T3, placing this case as T3 N0 which is Stage IIB if the contralateral nodules are disregarded since they were PET negative. The presence of such contralateral nodules affects the staging; with staging changing to M1a when there are contralateral lung nodules. However in this case these nodules were PET negative and could potentially have been parenchymal scars. There are two points to mention about these contralateral nodules. First, should they have indeed been tumors, they did not share the high metabolic rate profile of the nodules of the opposite side. Secondly, they were peripherally sited such that they could have been resected in the future by wedge resections in order to preserve lung parenchyma. This opinion varies from that of pulmonologists and oncologists who think that CUP is automatically best treated with chemotherapy radiotherapy and/or enrollment in a clinical trial for treatment with, for example, monoclonal antibodies such as pembrolizumab (22).
Survival rates
Patients with surgical resectable lung pathology fall into the 20% of CUP identified as having better prognosis, see Table 1; these carry a 15% 5-year survival and a median survival of 3 years, compared to the unfavorable subset that carries a dismal mean survival of 6 months (8). The survival rate associated with pathological staging is important, since that for T3 carries a 5-year survival rate of 22% similar to T4 disease; whilst the 5-year survival rate of M1a is 3% like other intrathoracic metastatic disease (23).
Conclusions
Diverging opinions between medical specialties with differing staging ethos may lead to varying treatments being offered to patients with CUP that may result in widely dissimilar outcomes. Better communication and in-depth discussions are recommended in multi-disciplinary team meeting when reviewing complex and unusual cases such as CUP.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.02.04). MS serves as an unpaid Associate Editor of Shanghai Chest. The other authors have other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Collado Martín R, García Palomo A, de la Cruz Merino L, et al. Clinical guideline SEOM: cancer of unknown primary site. Clin Transl Oncol 2014;16:1091-7. [Crossref] [PubMed]
- Fizazi K, Greco FA, Pavlidis N, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v133-8. [Crossref] [PubMed]
- Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 2009;69:271-8. [Crossref] [PubMed]
- Moran S, Martinez-Cardús A, Stergios Boussios S, et al. Precision medicine based on epigenomics: the paradigm of carcinoma of unknown primary. Nat Rev Clin Oncol 2017;14:682-94. [Crossref] [PubMed]
- Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med 2014;371:757-65. [Crossref] [PubMed]
- Rubin BP, Skarin AT, Pisick E, et al. Use of cytokeratins 7 and 20 in determining the origin of metastatic carcinoma of unknown primary, with special emphasis on lung cancer. Eur J Cancer Prev 2001;10:77-82. [Crossref] [PubMed]
- Golfinopoulos V, Pentheroudakis G, Salanti G, et al. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev 2009;35:570-3. [Crossref] [PubMed]
- Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: A clinical puzzle for the oncologists. J Adv Res 2015;6:375-82. [Crossref] [PubMed]
- Pavlidis N, Briasoulis E, Hainsworth J, et al. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990-2005. [Crossref] [PubMed]
- Lin WY, Hsu WH, Lin KH, et al. Role of preoperative PET-CT in assessing mediastinal and hilar lymph node status in early stage lung cancer. J Chin Med Assoc 2012;75:203-8. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193-203.
- Burglin SA, Hess S, Høilund-Carlsen PF, et al. 18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary. A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6713 [Crossref] [PubMed]
- Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009;19:731-44. [Crossref] [PubMed]
- Gallamini A, Zwarthoed C, Borra A. Positron Emission Tomography (PET) in oncology. Cancers (Basel) 2014;6:1821-89. [Crossref] [PubMed]
- Moses WW. Fundamental limits of spatial resolution in PET. Nucl Instrum Methods Phys Res A 2011;648:S236-40. [Crossref] [PubMed]
- Erdi YE. Limits of tumor detectability in nuclear medicine and PET. Mol Imaging Radionucl Ther 2012;21:23-8. [Crossref] [PubMed]
- Cheran SK, Nielsen ND, Patz EF Jr. False-negative findings for primary lung tumors on FDG positron emission tomography: staging and prognostic implications. Am J Roentgenol 2004;182:1129-32. [Crossref] [PubMed]
- Lim JS, Yun MJ, Kim MJ, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics 2006;26:143-56. [Crossref] [PubMed]
- Kandalaft PL, Gown AM. Practical applications in Immunohistochemistry. Carcinoma of unknown primary site. Arch Pathol Lab Med 2016;140:508-23. [Crossref] [PubMed]
- Rekhtman N, Bishop J. Quick reference handbook for surgical pathologists. Berlin: Springer-Verlag Berlin and Heidelberg Gmbh. 2011. p180.
- Oien KA, Dennis JL. Diagnostic work-up of carcinoma of unknown primary: from immunohistochemistry to molecular profiling. Ann Oncol 2012;23:x271-7. [Crossref] [PubMed]
- Study of Pembrolizumab and Concurrent Radiation in Patients With Previously Treated Carcinoma of Unknown Primary. Bethesda, MD: National Institutes of Health. Updated 9/21/2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03396471 Accessed 2/13/2019.
- UyBico SJ. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010;30:1163-81. [Crossref] [PubMed]
Cite this article as: Casha AR, Said-Huntingford I, Mizzi A, Gauci M, Scarci M. Management options for pulmonary nodules with cancer of unknown primary. Shanghai Chest 2019;3:17.


