Left-sided approach for robotic thymectomy: technical tips, advantages and drawbacks
IntroductionOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
Ashton and colleagues were one of the first to report a totally robotic approach to thymectomy in 2003 (1). Their right-sided approach was chosen due to the increased size of the right pleural space and superior visualization of the innominate vein and superior vena cava (1). The right-sided approach continued to be the preferred approach method for some time, in part due to the number of intraoperative cardiac injuries that were occurring from left-sided approaches in inexperienced, poorly trained hands. Now increasingly there has been improved safety with video-assisted thoracoscopic surgery (VATS) and robotic-assisted thymectomy.
Indications/contraindicationsOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
Virtually any patient who qualifies for an open procedure can undergo a robotic procedure. We have successfully removed tumors up to 13 cm robotically. Additionally, we have found the robotic approach to be safe in patients with prior radiation, chemotherapy, immunotherapy, and those with pericardial invasion required resection of part or all of innominate vein. Robotic thymectomy is appropriate for patients with myasthenia gravis with or without a thymoma as well as benign and malignant thymic tumors. The most important indication for surgery is an experienced robotic surgeon. Generally, the only relative contraindications to robotic thymectomy are invasion of the great vessels of the heart and very large tumor size >13 cm, but this is still not an absolute contraindication.
Preoperative evaluationOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
For any patient who is a candidate for robotic thymectomy, a thorough history and physical exam is essential. Pulmonary function testing and cardiac stress testing should be pursued as indicated. Computed tomography (CT) scan of the chest is generally all that is required for preoperative imaging. We recommend IV contrast as it can help with assessment of the innominate vein and superior vena cava. It is important to visualize a fat plane between the mass and vessels, as this signals that a robotic thymectomy can be attempted. If no fat plane is visualized or if the major vessels are clearly involved, we recommend thymectomy via median sternotomy with consideration for cardiopulmonary bypass. However, experienced surgeons may still consider robotic exploration first. Additionally, if a segment of lung appears to be involved, robotic wedge resection can be attempted.
For the myasthenic patient, it is critical for the surgeon to evaluate any current symptoms including ptosis, diplopia, dysarthria, dysphagia, dyspnea, difficulty chewing, slurred speech and fatigability. Surgeons should also ensure that myasthenic patients undergo the appropriate serologic and electrophysiologic evaluation and treatment prior to surgery. There remains no indication for urgent or emergent thymectomy during a myasthenic crisis—medical management should be pursued and elective thymectomy can be scheduled once resolved.
Patient and port positioningOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
The da Vinci® surgical system (Intuitive Surgical Inc., Sunnyvale CA, USA) is the only FDA-approved robotic system for lung surgery currently, and both the Si and Xi systems are available. We will describe our approach using the Xi system as it has several advantages including thinner and longer instrument arms, the ability to camera-hop to any arm/port, and the flexibility to approach the patient bed from multiple directions.
While initially we described robotic thymectomy via a right-chest approach (2), we now prefer a left-sided approach for most patients.
First, we insert a right-sided double lumen endotracheal tube to facilitate left lung collapse. Care is taken to position and tape the tube projecting cranially to avoid interference with the robotic arms.
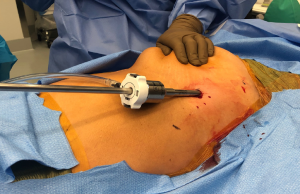
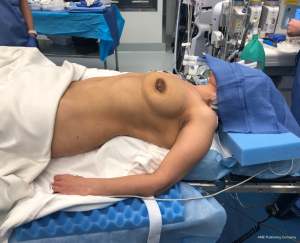
Perhaps the most important aspect of robotic thymectomy—especially on the left side—is the safety of entry of the ports to avoid cardiac injury. This means careful positioning—as shown in Figure 1—the patient is put with the left shoulder blade almost off the operating room table with a small bump/gel pad underneath the patient’s left shoulder blade and back. This bumps the left chest up and the left arm down which is placed on an arm board below the level of the patient’s hip. The arm is carefully secured and the patient is prepped and draped in normal sterile fashion.
The robotic ports need to be carefully marked out. We use the midline of the sternum and then go at least one rib below. If there is a large pendulous breast or a well-developed pectoralis major muscle, the left breast tissue is pulled medially by the assistant towards the right chest and the incision is made outside of the breast tissue but at the very curvilinear aspect of the contour of the left breast as shown in Figure 2. The camera port is placed first and is inserted carefully to avoid injury to the lung or to the heart. In this area, cardiac injury should be almost impossible, however it is incumbent on the surgeon to look at the preoperative imaging to ensure there has not been left cardiac deviation secondary to pathology or anatomic variance.
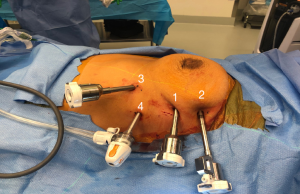
Once the camera port is inserted, carbon dioxide is insufflated in a special access port at a pressure of 7 mmHg and a flow of 20 L/minute (ConMed AirSeal®, Utica NY). The left internal mammary vein is seen where it empties in to the right innominate vein. In the right chest, this anatomy is more easily identified than in the left, and it is very important that the uppermost port is placed at least one rib below that level—if not it makes dissection of the superior thymic horns difficult to impossible. Rib spaces are then followed laterally with the camera, and the second port is placed. Importantly, an extra-long robotic trocar is used as shown in Figure 3. The camera is then placed into this port. A third port is placed in a gentle V-like slope low in the chest as shown in Figure 3. This port low in the chest but also lateral and inferior to the left inferior thymic horn to give the left hand the ability to maneuver and dissect the left inferior thymic horn (which is mostly fat) off of the tip of the pericardium.
After these three ports are placed, we use a 5-mm access port in all patients. If the tumor to be removed is greater than 6–7 cm, it is important that the access port is then extended into the left lower robotic port, and they should be placed over the same rib. If the tumor is smaller, the access port should be placed one or two ribs lower as shown in Figure 3. A more detailed video of port placement can be viewed here “Left Thymectomy Port Placement” (https://www.youtube.com/watch?v=smfsWMBxf6M). Once the ports are placed, the Xi robot is docked from the patient’s left side.
Operative steps and technical tipsOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
We use a Cadiere grasper in the left robotic hand, and a thoracic bipolar dissector in the right robotic hand. We usually prefer removing the ipsilateral inferior thymic horn first—thus in the left chest, start by dissecting the left inferior thymic horn. Often in the adult there is a large amount of fatty tissue. We have come to prefer dissecting this tissue and moving en masse over the top of the left upper lobe in the chest for removal later.
As soon as the left inferior horn is removed, the diaphragm should be visualized and any further fatty tissue should be removed from the diaphragm and pericardium. The left pleura is then opened. We prefer to open the left pleura around the mid-chest, but it may be opened lower. Once this is completed, blunt dissection can be used to identify the right internal mammary vein. Dissection should continue along the right internal mammary vein superiorly. Before completing this, the right inferior thymic horn should be removed. This can be difficult, as there is a large amount of fat and this step requires retraction by the bedside assistant as well as by the operating surgeon’s left hand as shown in our video link “Left Robotic Thymectomy” (https://youtu.be/7t_BOchB10E).
Once the left inferior horn is removed off the pericardium and diaphragm, attention is turned to bluntly dissecting the left pleural off Sibson’s fascia, exposing the entire right internal mammary vein where it empties into the right innominate vein. There is often a bit of adipose tissue that should be resected. The right phrenic nerve should be visualized while approaching the diaphragm, at which point the thymus can be lifted up to see where it ends. There is a small opening in the pericardium and pleura, and the phrenic nerve is just beneath it. This can often be seen in young adults or children with well-developed thymus glands. If it is difficult to find the right phrenic nerve, a 30-degree down camera may be used, the CO2 insufflation can be decreased, or—most importantly —indocyanine green (ICG) can be given intravenously to illuminate the bilateral phrenic veins that flank the phrenic nerve in both chests. Decreasing the CO2 insufflation is a nice trick because it brings the pericardium up into the chest and allows for improved visualization of the phrenic nerves.
At this point, we then prefer to dissect the right superior horn next, now that the entire mammary vein can be seen due to the innominate vein emptying into it. However, another option is to dissect along the left phrenic nerve up high into the mediastinum. Importantly, there are two places of ectopic thymic tissue that need to be removed—this is why we prefer a left-sided thymectomy in any patient with myasthenia gravis or a thymoma (since up to 15% of patients can develop postoperative myasthenia gravis after resection). The areas of ectopic thymic tissue are the aberrant/residual tissue underneath the innominate vein and aberrant/residual tissue that exists in the aortopulmonary window. We prefer to lift the left phrenic nerve up gently and dissect the tissue underneath. Some surgeons advocate removal of lymph nodes within the aortopulmonary window although there is a paucity of data on this subject.
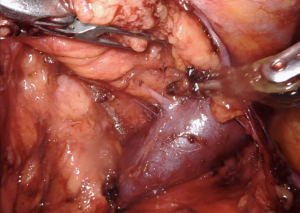
Once the two ectopic areas are completely freed, we then dissect along the inferior-lateral aspect of the innominate vein. Dissection should occur along the top of the vein, and caution should be taken around small branches of thymic vein that run anteriorly (Figure 4). These branches can be cauterized and clipped. The entire innominate vein should be dissected out and the right internal mammary vein should be visualized emptying into the innominate vein. Now, the two separate parts of the operation are connected. The only remaining steps are to dissect the superior thymic horns.
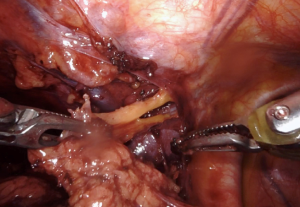
We always start on the contralateral superior thymic horn first. Importantly, the bedside assistant should bring a suction device into the chest and gently retract the innominate vein inferiorly to help facilitate dissection, as again shown in our video “Left Robotic Thymectomy” (https://youtu.be/7t_BOchB10E). Once the entire right superior horn is resected, now the bedside assistant can grab the thymic tissue and move it into the right chest and let the weight of the organ provide your retraction. The bedside assistant can again grab the thymic tissue and maneuver it down into the right chest, continuing to provide retraction (Figure 5). The robotic hands should be under the assistant’s retractor, and the left superior thymic horn should be dissected. Then, the assistant switches hands from a retractor pulling the thymus to a suction device holding the innominate vein down and the entire right superior horn is dissected. The remaining tissue that has been dissected should be placed in the left upper chest. Hemostasis is ensured and the specimen should be placed in a bag through the most inferior left-handed port, which requires upstaging to a 12-mm port. We do not routinely use a chest tube or drain, instead we use a long metal Yankauer suction catheter to suck air out of the chest right before the operation concludes. The wounds are then closed in layers.
Advantages and drawbacksOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
Adequate retraction is the key to completely bring down the superior thymic horns and is critical for complete thymectomy. This is essential especially in the case of myasthenia gravis. We have found it most helpful to alternate dissection above and below the superior horns to free it from the innominate vein and upper mediastinum.
The most feared vascular complication of thymectomy is injury to the innominate vein. A small injury may be stopped with pressure using a rolled up sponge and topical hemostatic agents. However, a larger injury should be addressed with packing if possible. Otherwise, the bedside assistant should apply pressure with a rolled up sponge while undocking the robot and preparing for sternotomy.
The most common technical issue is the robotic arm hitting the patient’s shoulder in both a left and right-sided approach. Refer to our positioning tips above and keep the patient’s shoulder as low as possible. We have found that placing the port more anteriorly gives more clearance from the shoulder when the robotic arm is directed toward the sternum. Using a longer robotic trocar is also helpful.
ResultsOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
From January 2009 to November 2018, we performed 185 robotic thymectomy procedures—104 (56.2%) via the right sided-approach and 81 (43.8%) by the left-sided approach. Of these patients, approximately 25% (n=48) were diagnosed with myasthenia gravis. Patient demographics are further detailed in Table 1. There were no open conversions and all patients had an R0 resection. Operative time was a median of 82 minutes (range, 33–289 minutes) and estimated blood loss remained low at a median of 10 mL (range, 5–200 mL). Median length of stay was 1 day (range, 0–6 days). The remainder of patient outcomes can be found in Table 2. There were no 30- or 90-day mortalities.
Table 1
| Variable | Patients undergoing robotic thymectomy |
|---|---|
| Age years, median [range] | 55 [14–88] years |
| Sex, n (%) | |
| Male | 104 (56.2) |
| Female | 81 (43.8) |
| Ethnicity, n (%) | |
| White | 140 (75.7) |
| Black | 39 (21.1) |
| Asian | 5 (2.7) |
| Hispanic | 1 (0.5) |
| BMI, median (range) | 28.9 (15.3–58.5) kg/m2 |
| Smoking history | |
| Yes, n (%) | 68 (36.8) |
| Pack-years (median, range) | 20 (0.5–225) |
| Months quit (median, range) | 180 (0.5–564) |
| Current smoker, n (%) | 22 (11.9) |
| Myasthenia gravis, n (%) | 48 (25.8) |
| Comorbid conditions (N) | |
| History of cancer | 23 |
| History of chemotherapy | 18 |
| History of radiation | 11 |
| Immunocompromised or currently taking corticosteroids | 45 |
| Hypertension | 72 |
| Congestive heart failure (CHF) | 5 |
| Coronary artery disease (CAD) or stent | 15 |
| Peripheral vascular disease (PVD) | 7 |
| Prior cardiothoracic surgery | 22 |
| History of cerebrovascular accident/stroke | 3 |
| Diabetes | 17 |
| Hyperlipidemia | 33 |
| Chronic obstructive pulmonary disease (COPD) | 9 |
| History of chronic pain (currently taking gabapentin, pregabalin or opioids) | 15 |
| Serum creatinine, median (range) | 0.9 (0.3–1.9) |
| Hemoglobin, median (range) | 13.6 (7.9–17.6) |
| ECOG/WHO/Zubrod Score (median) | 1 |
| Forced expiratory volume in 1 s, median (range) | 86% (41–124%) |
| Diffusing capacity of lung for carbon monoxide, median (range) | 81% (44–133%) |
ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization.
Table 2
| Variable | Patients who had a robotic thymectomy |
|---|---|
| Approach, n (%) | |
| Right-sided | 103 (55.7) |
| Left-sided | 82 (44.3) |
| Conversion to open | 0 |
| Nodule size in largest dimension, median (range) | 3.5 (0.9–13) cm |
| SUVmax of resected mass, median (range) | 4.75 (0–18) |
| R0 resection, n (%) | 185 (100%) |
| Final pathology | |
| Benign (normal thymus tissue, lipoma, cyst, granuloma) | 57 |
| Thymoma | 56 |
| Thymic hyperplasia | 42 |
| Lymphoma | 8 |
| Ectopic parathyroid | 5 |
| Ectopic thyroid | 5 |
| Metastatic disease | 5 |
| Thymic carcinoma | 4 |
| Cystic lymphangioma | 1 |
| Langerhan’s histiocytosis | 1 |
| Cystic teratoma | 1 |
| Operative time, median (range) | 82 [33–289] minutes |
| Estimated blood loss, median (range) | 10 [5–200] mL |
| Hospital length of stay, median (range) | 1 (0–6) days |
| Minor complications (N) | |
| Prolonged pneumothorax | 1 |
| Subcutaneous emphysema | 1 |
| Brachial plexopathy | 1 |
| New-onset postoperative atrial fibrillation | 2 |
| Pleural effusion requiring chest tube drainage | 1 |
| Major complications (N) | |
| Reintubation | 1 |
| Bleeding requiring reoperation | 1 |
| Myocardial infarction | 1 |
| Blood transfusion required (N) | 0 |
| Mortality (30- and 90-day) (N) | 0 |
SUV, standardized uptake value.
Robotic thymectomy offers distinct perioperative and postoperative advantages when compared to the traditional sternotomy approach. Less intraoperative blood loss, shorter length of hospital stay and improved postoperative patient quality of life have been demonstrated in multiple studies (3-5). While some studies note a longer operative time with the robotic approach (4), we feel this is not consistent among all surgeons and improves with increased experience (6). Additionally, large tumors and thymomas should not dissuade a surgeon from the robotic approach, as they can still be resected safely (7). The advantage of robotic thymectomy over VATS approach is less clear, however we cannot argue with the shorter robotic learning curve and increased degrees of movement and freedom that robotic arms provide (8-10). We advise each surgeon to tailor their approach and technique to their own comfort and skill level (11).
Specifically for non-thymomatous patients with myasthenia gravis, robotic thymectomy offers the potential for complete disease remission, and may improve patient quality of life vs. prolonged immunosuppressive medical therapy (12-17). Additionally, several studies showed a small difference in myasthenia remission rates between traditional sternotomy, VATS and robotic approaches for thymectomy, further advocating for a minimally-invasive approach for these patients (17-21).
ConclusionsOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
In summary, robotic thymectomy is safe with minimal perioperative morbidity and can offer excellent results for myasthenia gravis. We now prefer a left-sided approach for most patients.
AcknowledgmentsOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giuseppe Marulli) for the series “Robotic Mediastinal Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: The series “Robotic Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Cerfolio discloses relationships with Bovie, Community Health Services, Covidien/Medtronic, C-SATS, Davol/Bard, Ethicon, Google/Verb, Intuitive Surgical, KCI/Acelity Company, Myriad Genetics, Pinnacle, ROLO-7 Consulting Firm and TEGO Corporation. Dr. Ferrari-Light has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Indications/contraindications
- Preoperative evaluation
- Patient and port positioning
- Operative steps and technical tips
- Advantages and drawbacks
- Results
- Conclusions
- Acknowledgments
- Footnote
- References
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Wei B, Cerfolio R. Robotic thymectomy. J Vis Surg 2016;2:136. [Crossref] [PubMed]
- Gkouma A. Robotically assisted thymectomy: a review of the literature. J Robot Surg 2018;12:3-10. [Crossref] [PubMed]
- Marulli G, Comacchio GM, Schiavon M, et al. Comparing robotic and trans-sternal thymectomy for early-stage thymoma: a propensity score-matching study. Eur J Cardiothorac Surg 2018;54:579-84. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Casiraghi M, Galetta D, Borri A, et al. Robotic-assisted thymectomy for early-stage thymoma: a propensity-score matched analysis. J Robot Surg 2018;12:719-24. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Fok M, Bashir M, Harky A, et al. Video-Assisted Thoracoscopic Versus Robotic-Assisted Thoracoscopic Thymectomy: Systematic Review and Meta-analysis. Innovations (Phila) 2017;12:259-64. [Crossref] [PubMed]
- Hartwich J, Tyagi S, Margaron F, et al. Robot-assisted thoracoscopic thymectomy for treating myasthenia gravis in children. J Laparoendosc Adv Surg Tech A 2012;22:925-9. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Batirel HF. Minimally invasive techniques in thymic surgery: a worldwide perspective. J Vis Surg 2018;4:7. [Crossref] [PubMed]
- Diaz A, Black E, Dunning J. Is thymectomy in non-thymomatous myasthenia gravis of any benefit? Interact Cardiovasc Thorac Surg 2014;18:381-9. [Crossref] [PubMed]
- Yin DT, Huang L, Han B, et al. Independent long-term result of robotic thymectomy for myasthenia gravis, a single center experience. J Thorac Dis 2018;10:321-9. [Crossref] [PubMed]
- Keijzers M, de Baets M, Hochstenbag M, et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur J Cardiothorac Surg 2015;48:40-5. [Crossref] [PubMed]
- Ismail M, Swierzy M, Ruckert RI, et al. Robotic thymectomy for myasthenia gravis. Thorac Surg Clin 2014;24:189-95. vi-vii. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4; discussion 504-5. [Crossref] [PubMed]
- Renaud S, Santelmo N, Renaud M, et al. Robotic-assisted thymectomy with Da Vinci II versus sternotomy in the surgical treatment of non-thymomatous myasthenia gravis: early results. Rev Neurol (Paris) 2013;169:30-6. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Van Woerkom JM, et al. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. Ann Thorac Surg 2011;92:1018-22; discussion 1022-3. [Crossref] [PubMed]
- Zielinski M, Hauer L, Hauer J, et al. Comparison of complete remission rates after 5 year follow-up of three different techniques of thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2010;37:1137-43. [Crossref] [PubMed]
Cite this article as: Ferrari-Light D, Cerfolio RJ. Left-sided approach for robotic thymectomy: technical tips, advantages and drawbacks. Shanghai Chest 2019;3:3.