Pulmonary artery resections for lung cancer—when and how?
Introduction
Centrally-located lung cancer often requires a multidisciplinary approach with either induction or adjuvant therapy. Historically pneumonectomy was the surgical procedure routinely performed for these tumors. However, over the last three decades a large number of studies demonstrated an improved outcome with parenchymal sparing procedures when compared to pneumonectomy, with lower postoperative morbidity and mortality, guaranteeing complete resection (1,2).
Resection and reconstruction of the pulmonary artery (PA) may be considered one of the most challenging and beneficial procedures in thoracic surgery. In addition to lobectomy, whether associated or not to a sleeve resection of the bronchus, this procedure allows centrally located lung cancer complete resection, thus avoiding pneumonectomy (3).
Moreover, pneumonectomy is often associated to a serious postoperative impairment of cardiac (4) and lung function (5), and quality of life (6). For this reason, pneumonectomy is considered a disease itself, particularly when performed on the right side and after induction chemotherapy (7). Nowadays pneumonectomy is recommended only to achieve full oncological radicality when this cannot be obtained through parenchymal sparing procedures. Therefore, PA reconstruction should be carried out not only in case of cardiorespiratory impairment, but it should be always preferred when the procedure is technically feasible.
When performing a PA resection and reconstruction, patients need a preoperative clinical and radiological work-up not different from the other standard lung resection. This will vary according to the stage of disease, the patient’s performance status and each single center policy. At our center preoperative workup usually includes total body contrast computed tomography (CT) scan, pulmonary functions tests, cardiac evaluation, blood gas analysis and routine blood tests.
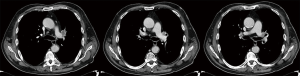
The primary indication for PA resection is infiltration of the origin of the lobar branches by primary lung cancer or by metastatic hilar lymph nodes (N1) (Figure 1A,B,C) with extracapsular extension or, after induction therapy, a desmoplastic reaction, scar tissue or fibrosis that linger on the site of the previous tumor infiltration. Thus, it could range from a partial infiltration of the vessel to a more extensive and even circumferential invasion that may require different resection and reconstruction techniques. PA reconstruction is more frequent on the left side, in association with upper lobectomy (70% of the cases) and with a right upper lobectomy (20%). Only in a 10% of the patients the procedure is performed on the main PA or in association with inferior lobectomy on both side (3).
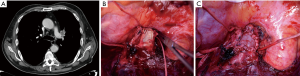
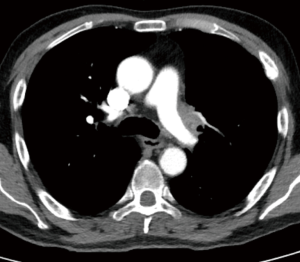
The vessel infiltration can anticipated at preoperative imaging but the true status can be assessed only intraoperatively (Figure 2). If only a marginal, limited infiltration of the PA branches’ origin is identified, a tangential resection with direct suture is performed. We consider this technique as a variation of standard lobectomy and it is usually not classified as a reconstructive procedure. When up to 30% to 40% of the circumference of the vessel is involved by the tumor, a patch reconstruction, usually with biological materials, can be performed. In case of a more extended degree of infiltration a circumferential resection is required and the reconstruction can be performed by end to end anastomosis or by the interposition of a prosthetic conduit (biological or synthetic) (8).
Operative technique
The operative technique includes two steps: the resection of the vessel and then the reconstruction of the PA.
Complete control of the proximal PA and distal PA is mandatory as well as both pulmonary veins (PVs) and the bronchus. The exposure of the PA can be more easily performed if the superior PV is completely dissected and retracted, but the transection of the vessel should be postponed until the feasibility of the procedure is established. Before clamping the main PA low dose intravenous heparin is administrated.
Over time we progressively modified the intra-operative management: we now tend to clamp the PA distally to the neoplastic infiltration, if possible. The intravenous dose of heparin is reduced to about 25 units/kg (1.500–2,000 units). The latter change contributes to prevent intra- and postoperative oozing, particularly from mediastinal lymphadenectomy, without any additional thrombosis events. At the end of the vascular reconstruction heparin is not reversed with protamine.
The proximal control of the main PA can be obtained both intra- or extrapericardially on a case by case basis particularly, on the left side the main PA is often involved close to its origin thus requiring an intrapericardial approach. In case of invasion of the interlobar artery only, it is possible to prepare it without opening the pericardium. On the right side usually the main PA is isolated extrapericardially, by retracting medially the superior vena cava.
After en bloc removal of the resected lobe with the portion of infiltrated PA the reconstruction phase may begin.
Patch reconstruction
When the tumor infiltrated only the lobar aspect of the PA for not more than 30–40% of the circumference, a patch reconstruction is indicated. The defect may be of limited to large dimension on the longitudinal axis of the vessel and its shape is usually stretched in this direction. For that reason, the size and shape of the patch is tailored intraoperatively on the PA defect itself.
Different materials have been proposed for patch reconstruction: synthetic [polytetrafluoroethylene (PTFE) graft] (9), autologous (pericardium and PV) and heterologous (bovine and porcine pericardium).
Autologous pericardium is fresh, unpreserved, available on both sides of the chest and it can be harvested during the surgical procedure; it is generally sufficient to cover even large defects, it is cost-free and perfectly biocompatible. It is usually harvested anteriorly to the phrenic nerve, without any need of reconstruction of the pericardial defect (10).
In order to facilitate the patch’s manipulation and suturing, intraoperative fixation by a glutaraldehyde-buffered solution is advisable (11). The fresh pericardium is fixed in diluted glutaraldehyde for few minutes, washed with saline and then adequately trimmed to the appropriate size; the patch’s epicardial surface stays inside the lumen of the vessel. This technique contributes to minimize the tendency of the harvested pericardium to curl and retract. The patch becomes more stiff, with a lower tendency to recoil during suturing and a lower risk of bleeding.
Although our choice remains the use of autologous pericardium, other biological options have been reported as venous patches: superficial femoral vein (12), azygos vein, saphenous vein, and, recently, the superior PV patches and bovine and porcine pericardium patches (13). Among these options, the azygos vein can be found only on the right side, while the saphenous vein and the superficial femoral vein need another surgical procedure to be harvested, both providing a little amount of tissue for the reconstruction. These procedures are also time consuming and may be traumatic for the patients. The superior PV can provide enough material for the reconstruction (14-16), but it is usually obtained, due to anatomical reasons, only when it is associated with left upper lobectomy; it is more difficult on right side. During the preparation for upper lobectomy, in case the extra-parenchymal portion of the PV is not infiltrated by and sufficiently far from the tumor, the vessel can prepared from the atrial confluence intrapericardially to its extrapericardial portion, up to proximal branches. The superior PV is then closed proximally, at the atrial confluence, by a TA stapler and is ligated distally at the extralobar origin of the branches; a 1.5 to 2.5 cm long conduit is obtained. After obtaining frozen sections of the specimen PV margins, the conduit can be trimmed to obtain a suitable PA patch oriented with the endovascular layer looking inside the artery (13).
The bovine pericardium has been used as a valid alternative to autologous pericardium over the years. It reduces the harvesting and trimming time allowing more regular margins, but it also shows very little elasticity; however the edges are stiffer and easy to suture. The porcine pericardium shows similar properties, but it is thinner and more flexible.
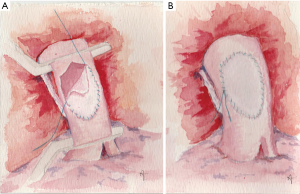
Whatever patch we choose, two stay sutures are usually placed at the opposite sites of the patch. Then, while the assistant stretches and holds the patch in place, a 5-0 monofilament non-absorbable suture is used to anastomose it (Figure 3A,B). The inferior stay suture is removed when the suture line reaches it.
The patency of the vessel is assessed after expansion of the residual lobe, to prevent kinking due to elevation of the parenchyma (3).
If a bronchial sleeve resection is required, the patch reconstruction is usually performed before the bronchial anastomosis to reduce the arterial clamping time.
End-to-end anastomosis
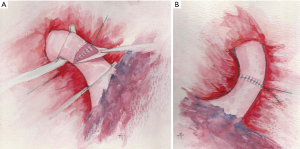
When more than one-half of the PA circumference is involved by the tumor, a circumferential resection is required with end-to-end anastomosis of the stumps. In this case, it is better to complete the bronchial anastomosis first, as the bronchial axis is shortened so that the two vascular stumps can be approximated with less tension and the surgeon can minimize manipulation. Usually, two key factors help to solve the size discrepancy of the two sides of the anastomosis: the elasticity of the PA and careful stiches placement. Nevertheless, it is important to obtain regular margins when transecting the PA on both sides to facilitate the caliber correction and to avoid stenosis. Another trick to reduce tension on the anastomosis after completion of the bronchial reconstruction is to elevate the lower lobe with a sponge while suturing the PA. The anastomosis is completed with a running 5-0 or 6-0 monofilament non-absorbable suture (Figure 4A,B). At the end of the anastomosis, the removal of the proximal clamp usually relieves the residual tension (17).
Prosthetic conduit reconstruction
Despite all the expedients mentioned before, if the distance between the PA stumps is judged excessive interposition of a prosthetic conduit is mandatory. This is an infrequent condition usually occurring on the left side, when a left upper lobe tumor extensively infiltrates the PA without the involvement of the corresponding lobar bronchus, thus not requiring a bronchial sleeve resection.
Over the years, different techniques and materials have been employed for this procedure. Although the use of PTFE has been reported with good results (18,19) as well as other synthetic materials, it might increase the risk of thrombosis, especially for a low-pressure vessel, and it may require long-term anticoagulation.
In the literature numerous biological materials have been proposed for PA conduit replacement: the autologous pericardium, the bovine pericardium (20,21) and, more recently, the cryopreserved allograft (PA or aorta) (22), the porcine pericardium (13) and the superior PV (14-16).
The pericardial conduit can be easily prepared by harvesting a relatively large pericardial patch that is trimmed in a rectangular shape and wrapped around a chest tube drainage with the epicardial surface inside; the longitudinal suture is completed with a running 6/0 monofilament non-absorbable material, creating an approximately 1.5–2 cm long conduit (20).
Berthet reported the use of cryopreserved allografts harvested from multi-organ donors, implanting both PA and thoracic aorta. The ABO blood group and human leucocyte antigen group biocompatibility were not required. The appropriate graft is selected on the basis of morphologic preoperative CT scan data (22).
Among the biological materials, the porcine pericardium has recently been used by our group. The conduit can be intraoperatively trimmed and wrapped on a chest tube or a syringe of the correct size (23) (Figure 5).

D’Andrilli proposed that an effective autologous conduit can be obtain with the superior PV (15,16). When the extra-lobar PV branches are free from tumor, the vessel can be used as a valid alternative for PA reconstruction. The similarity of the two vessels’ wall confers the perfect thick to the conduit, thus allowing an easy anastomosis. The PV conduit harvesting procedure has been previously described.
The appropriate length of the conduit should be tailored on the resected arterial segment. Usually, a 1.5- to 2.5-cm long conduit is sufficient enough to fill the gap between the vascular stumps.
The proximal anastomosis is initially completed with a running 5/0 monofilament non-absorbable suture and after a check of the conduit length, the distal one is completed with the same technique. Two points are crucial in completing the vascular replacement: the need to avoid an excessive conduit length that could lead to kinking of the vessel and the necessity to avoid any tension at the level of the anastomosis. The latter point can be achieved with the section of the inferior pulmonary ligament and, on the right side, by the opening of the pericardium around the inferior PV.
Reconstruction of the main PA
The involvement of the main PA is a rare condition caused by a proximal invasion of left hilar tumors (Figure 6). It usually required pneumonectomy. Median sternotomy is performed and the pericardium is opened. The main PA as well as the right and left PA branches are dissected free from the surrounding structures. Cardiopulmonary by-pass is instituted. The right PA is clamped and the involved vessel is removed en bloc with the left lung. The defect is repaired with a patch (3).
Perioperative management
When a sleeve lobectomy is associated with a PA reconstruction, it is recommended to perform the positioning of soft tissue between the bronchial and the vascular anastomosis. The interposition of a pedicled intercostal flap has been used by our group with excellent results; the intercostal artery supplies an ideal easily revascularization (24,25). The muscle flap is wrapped around the bronchial anastomosis to protect and revascularize the suture line and to prevent direct contact between the two structures; it is secured to the bronchus by interrupted absorbable 4-0 sutures. Other means of protection such as the pericardial flap, the pleural flap, or the mediastinal fat pad have also been proposed (2,26,27). In addition, in case of double sleeve lobectomy the use of low dose steroids during the early postoperative course has been suggested to reduced edema and improve healing (28).
When a biological patch or conduit is employed no long-term anticoagulation is recommend if no other disorders coexist; subcutaneous low-molecular weight heparin is administrated according to standard protocols of prevention of pulmonary embolism (21).
Outcome
Outcome usually depends on the choice of the most appropriate reconstruction procedure on a case by case basis. Patency, cardiac and pulmonary function, and completeness of resection are the keys for long-term survival.
When the procedure is correctly performed, complications could be anticipated in less than 5% of the patients; moderate leakage from the suture line, kinking or vessel thrombosis are the most frequent (3). Technical improvements and the widespread use of biological materials have significantly reduced thrombosis. Recently a rate of 0 to 2% of thrombosis requiring completion pneumonectomy has been reported (10,21,29). Contrast CT scan and three dimension (3D) volume rendering or an Angio-magnetic resonance are useful noninvasive tools to detect patency defect, both early and late in the postoperative course (30).
When PA reconstruction is associated to a bronchial sleeve lobectomy, bronchopleural or bronchovascular fistulas rarely occur. Intraoperative protection of the anastomosis (31) and prompt conservative treatment of the fistula reduces the risks of empyema and mortality (32,33).
It has been shown that, after PA reconstruction, the functional parameters are comparable with those reported for standard lobectomy (34-36). Our group previously showed that right heart morphology and function are also comparable with standard lobectomy (10).
These reconstruction lung sparing procedures can be performed also after induction chemotherapy (37,38). The procedure gained a widespread consensus for the treatment of centrally located lung cancer and a number of studies supported both functional and survival advantages when compared to pneumonectomy (1,2,39,40). Initial concerns on the PA reconstruction were raised due to its long-term patency and effects on cardiopulmonary function, and, later, on its oncological accuracy and survival. Nevertheless, over the past thirty years a number of studies reported continuous enhancements of surgical technique, reduction in complication rate and improvements in the survival rate (10,18,34,41). Table 1 reports several studies over last decade and it includes series with more than 20 patients receiving PA resection and reconstruction.
Table 1
| References | Patients (concomitant bronchial sleeve) | Type of PA reconstruction | Complications (%) | Technical complications (n) | Mortality (%) | 5-year overall survival | Disease free survival (%) |
|---|---|---|---|---|---|---|---|
| Cerfolio et al. 2007 (25) | 42 [6] | TR [31] | 26 | 0 | 2.3 | 60 | NR |
| Patch [7] | |||||||
| EE [4] | |||||||
| Conduit (–) | |||||||
| Alifano et al. 2009 (26) | 93 [23] | TR [88] | 29 | NR | 5.4 | 39.4 | NR |
| Patch [3] | |||||||
| EE [2] | |||||||
| Conduit (–) | |||||||
| Venuta et al. 2009 (21)ǂ | 105 [65] | Patch [55] | 28.5 | 1 | 0.95 | 44 | 43.1 |
| EE [47] | |||||||
| Conduit [3] | |||||||
| Berthet et al. 2013 (22)* | 32 [3] | Patch [2] | 50 | 1 | 0 | 66.7 | NR§ |
| EE [20] | |||||||
| Conduit [10] | |||||||
| Ma et al. 2013 (29) | 118 [41] | TR [26] | 52.2 | 1 | 0 | 50.2 | NR |
| Patch [51] | |||||||
| EE [41] | |||||||
| Conduit (–) | |||||||
| Galetta et al. 2015 (19) | 150 [56] | TR [80] | 30.7 | 0 | 3.3 | 50.4 | 49.1 |
| Patch [66] | |||||||
| EE (–) | |||||||
| Conduit [4] | |||||||
| D’Andrilli et al. 2017 (16) | 24 [1] | Patch [4] | 29.1 | 0 | 0 | 69.9 | 52.7 |
| EE (–) | |||||||
| Conduit [20] |
Figures calculated from data reported in article. NR, not reported. *, data collected only on patient underwent pulmonary artery replacement; ǂ, survival analysis calculated excluding 3 patients underwent pneumonectomy and patch reconstruction under CPB; §, reported estimated median disease free survival =42 months. TR, tangential resection; EE, end-to-end anastomosis.
The role of reconstructive parenchymal sparing procedures, albeit proved to be advantageous for survival of stage I and II patients, still produce uncertain effects in case of stage III lung cancer, particularly N2 disease. When an analysis of the survival rate is performed for the PA reconstruction, both stage by stage and on the impact of the nodal status on survival, its oncologic reliability is found to be comparable to that of a large series of bronchial sleeve resection studies as well as to those found within the lung cancer literature (10,21,41).
In conclusion PA reconstruction, when correctly performed is a safe, feasible and oncologically effective procedure.
Acknowledgments
The authors acknowledge Matteo Sala for figures editing and M. Alessandra Trivero for watercolor paintings.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Alper Toker and Alan Sihoe) for the series “Extended resections for lung cancer” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.10.01). The series “Extended resections for lung cancer” was commissioned by the editorial office without any funding or sponsorship. FV serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-smallcell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Rendina EA, Venuta F. Reconstruction of the pulmonary artery. In: Patterson GA, Cooper JD, Deslauriers J, et al. editors. Origins of Pearsons Thoracic & Esophageal Surgery. 3rd edition. Philadelphia, PA: Churchill Livingstone, Elsevier, 2008:909-22.
- Venuta F, Sciomer S, Andreetti C, et al. Long-term Doppler echocardiographic evaluation of the right heart after major lung resections. Eur J Cardiothorac Surg 2007;32:787-90. [Crossref] [PubMed]
- Melloul E, Egger B, Krueger T, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg 2008;7:986-9. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg. 2003;76:1782-8. [Crossref] [PubMed]
- Venuta F, Anile M, Diso D, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007;31:714-7. [Crossref] [PubMed]
- Venuta F, Ciccone AM. Reconstruction of the pulmonary artery. Semin Thorac Cardiovasc Surg 2006;18:104-8. [Crossref] [PubMed]
- Obuchi T, Miyahara S, Higuchi T, et al. Prognosis of patients after pulmonary arteryplasty for non-small cell lung cancer. Thorac Cardiovasc Surg 2009;57:484-8. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001; discussion 1001-2. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehydepreserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. [Crossref] [PubMed]
- Kotzampassakis N, Corpataux JM, Pasche P, et al. Superficial femoral vein as substitute for pulmonary artery reconstruction after resection for bronchovascular fistula. J Thorac Cardiovasc Surg 2008;136:525-7. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. J Thorac Dis 2016;8:S168-80. [PubMed]
- Cerezo F, Cano JR, Espinosa D, et al. New technique for pulmonary artery reconstruction. Eur J Cardiothorac Surg 2009;36:422-3. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Pulmonary artery reconstruction with pulmonary vein conduit for lung cancer: medium-term results. Ann Thorac Surg 2014;98:990-5. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Ciccone AM, et al. Long-segment pulmonary artery resection to avoid pneumonectomy: long-term results after prosthetic replacement. Eur J Cardiothorac Surg 2017; [Epub ahead of print]. [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Lung conservation techniques: bronchial sleeve resection and reconstruction of the pulmonary artery. Semin Surg Oncol 2000;18:165-72. [Crossref] [PubMed]
- Read RC, Ziomek S, Ranval TJ, et al. Pulmonary artery sleeve resection for abutting left upper lobe lesions. Ann Thorac Surg 1993;55:850-4; discussion 853-4. [Crossref] [PubMed]
- Galetta D, Borri A, Gasparri R, et al. Surgical Techniques and Long-Term Results of Pulmonary Artery Reconstruction in Patients With Lung Cancer. Ann Thorac Surg 2015;100:1196-202; discussion 1202. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Reconstruction of the pulmonary artery by a conduit of autologous pericardium. J Thorac Cardiovasc Surg 1995;110:867-8. [Crossref] [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Berthet JP, Boada M, Paradela M, et al. Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg 2013;146:1191-7. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-S881. [Crossref] [PubMed]
- Rendina EA, Venuta F, Ricci P, et al. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg 1994;107:1251-4. [PubMed]
- Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 1976-7.
- Alifano M, Cusumano G, Strano S, et al. Lobectomy with pulmonary artery resection: morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg 2009;137:1400-5. [Crossref] [PubMed]
- Tsuchiya R. Bronchoplastic techniques. In: Patterson GA, Deslauriers J, Lerut A, et al. editors. Pearson’s Thoracic and Esophageal Surgery. 2nd ed. Philadelphia, PA: Churchill Livingstone, 2002:1005.
- Rendina EA, Venuta F, Ciriaco P, et al. Bronchovascular sleeve resection. Technique, perioperative management, prevention, and treatment of complications. J Thorac Cardiovasc Surg 1993;106:73-9. [PubMed]
- Ma Q, Liu D, Guo Y, et al. Surgical techniques and results of the pulmonary artery reconstruction for patients with central non-small cell lung cancer. J Cardiothorac Surg 2013;8:219. [Crossref] [PubMed]
- Ciccone AM, Venuta F, D'Andrilli A, et al. Long-term patency of the stapled bovine pericardial conduit for replacement of the superior vena cava. Eur J Cardiothorac Surg 2011;40:1487-91; discussion 1491. [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Intercostal pedicle flap in tracheobronchial surgery. Ann Thorac Surg 1996;62:630-1. [Crossref] [PubMed]
- Menna C, Poggi C, Ibrahim M, et al. Coated expandable metal stents are effective irrespective of airway pathology. J Thorac Dis 2017;9:4574-83. [Crossref] [PubMed]
- Andreetti C, D'Andrilli A, Ibrahim M, et al. Submucosal injection of the silver-human albumin complex for the treatment of bronchopleural fistula. Eur J Cardiothorac Surg 2010;37:40-3. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Tscholl D, et al. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg 2005;79:1147-52; discussion 1147-52. [Crossref] [PubMed]
- Shrager JB, Lambright ES, McGrath CM, et al. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg 2000;70:234-9. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Sleeve Lobectomy Versus Standard Lobectomy for Lung Cancer: Functional and Oncologic Evaluation. Ann Thorac Surg 2016;101:1936-42. [Crossref] [PubMed]
- Ricci C, Rendina EA, Venuta F, et al. Reconstruction of the pulmonary artery in patients with lung cancer. Ann Thorac Surg 1994;57:627-32; discussion 632-3. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Nagayasu T, Yamasaki N, Tsuchiya T, et al. The evolution of bronchoplasty and broncho-angioplasty as treatments for lung cancer: evaluation of 30 years of data from a single institution. Eur J Cardiothorac Surg 2016;49:300-6. [Crossref] [PubMed]
Cite this article as: Poggi C, Mantovani S, Pecoraro Y, Carillo C, Bassi M, Bruschini P, Pagini A, Amore D, Rendina EA, Diso D, Venuta F, Anile M. Pulmonary artery resections for lung cancer—when and how? Shanghai Chest 2018;2:79.