Pericardial-peritoneal window for malignant pericardial effusion
Introduction
The pericardium is a double-layered sac (visceral and parietal layers) that envelops the heart and proximal great vessels and contains 20–50 mL of pericardial fluid. Pericardial effusion is a pathological accumulation of fluid in the pericardial cavity (1). The condition is common in patients with cancer and can be due to metastatic involvement of the pericardium, inflammatory or infectious conditions, heart failure, complications of cancer treatments and, rarely, primary pericardial tumors (2).
The optimal treatment of malignant pericardial effusion for patients who develop tamponade or a pre-tamponade clinical condition remains controversial. Nonetheless, it should ensure complete and permanent drainage with minimal discomfort and risk to the patient, and also provide sufficient histologic, cytologic and microbiologic material for diagnostic study (3).
The first reported the use of the subxiphoid approach for drainage of the pericardial cavity was by Napoleon’s surgeon Larrey in 1829 (4). Since then the subxiphoid pericardial window or pericardiostomy has been commonly used to treat pericardial effusion (3). Alternative surgical techniques for treating pericardial effusion include pericardiectomy or pericardial window through sternotomic, thoracotomic or video-assisted approaches (5-7).
The aim of this study was to analyze the technical aspects, preoperative imaging assessment, clinical outcomes, morbidity and mortality, cytohistological data and median overall survival (OS) in a retrospective series of patients undergoing pericardial-peritoneal window for malignant pericardial effusions in the last decade.
Methods
Data were collected prospectively and entered into our institutional general thoracic database at the point of care and reviewed and double-checked retrospectively. Twenty consecutive patients with malignant pleural effusion undergoing pericardial-peritoneal window from 2006 to 2017 were enrolled in the present study.
Written informed consent to undergo the procedure and the use of clinical and imaging data for scientific and/or educational purposes was obtained from all patients before the operation according to European Union and Italian legislation.
Data were collected on sex, age, preoperative ultrasound and computed tomography (CT) findings, anatomic district, histology and pathological stage of the neoplasm, intraoperative findings and additional surgical procedures needed. Further information included total postoperative complications, 30-day mortality rate, specific pulmonary and cardiac complications, ICU admission and hospital stay, and median OS.
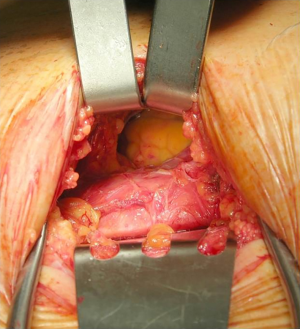
Pericardio-peritoneal window was performed under general anesthesia and single lumen orotracheal intubation and ventilation. A subxiphoid longitudinal approach was used to expose the xiphoid process and the upper abdomen. The pericardium was opened in the anteroinferior surface while the peritoneum was opened in its apical part. The retrosternal central part of the diaphragm was incised to create a transdiaphragmatic communication between the pericardium and the peritoneum. The edge of the opened pericardium, diaphragm and peritoneum were sutured by interrupted non-absorbable stitches on both sides, to leave the tunnel open. An intrapericardial drain was left inside and removed 24 hours after the procedure if no bleeding or major effusion was observed (Figures 1,2).
Complications were classified according to the Thoracic Morbidity and Mortality (TM&M) classification system as Minor (Grades I and II) and Major (Grade IIIa, b; Grade IVa, b; Grade V) (8).
Statistical methods
Summary statistics of clinicopathological characteristics of patients and age at the date of surgery were produced and tabulated as counts and either percent or mean, standard deviation (SD), min and max for categorical and continuous variables respectively. OS was defined as the time from surgery date to the date of death and estimated by the Kaplan-Meier method.
Postoperative death was defined as the 30-day mortality or longer if mortality occurred during hospitalization.
Survival time for patients still alive at the last follow-up date were considered censored. Median follow-up duration was estimated by the inverted Kaplan-Meier method.
Results
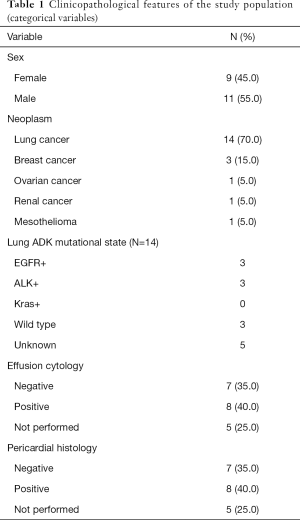
From 2006 to 2017, 20 consecutive patients underwent pericardial-peritoneal window to treat malignant pleural effusion. Eleven patients were male and nine were female; mean age was 63 years (range, 29–81 years); 14 patients had lung cancer, 3 breast ductal carcinomas, 1 papillary serous ovarian adenocarcinoma, 1 renal cell carcinoma and 1 malignant mesothelioma. Among the 14 patients with lung cancer, eleven had adenocarcinoma, one adenosquamous carcinoma, one sarcomatoid and one non-small cell lung cancer not otherwise specified. FISH was performed in nine patients with lung adenocarcinoma and mutational status was assessed as follows: three patients presented EGFR mutations; three presented K ras mutation and three were wild type; we did not observe any ALK translocations (Table 1).
Full table
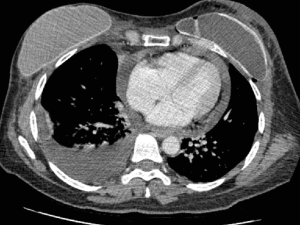
Preoperative cardiac ultrasound disclosed a mean ejection fraction of 60% (range, 38–70%) and a mean telediastolic volume of 87.9 mL (range, 48–175 mL). Mean quantification of pericardial effusion by ultrasound—defined as the thickness of the uniform echo-free space around the heart—was 18 mm (range, 13–23 mm) while by CT scan—considered as the maximum detachment between the pericardium and the heart—it was 21.25 mm (range, 13.7–39.3 mm).
Cytology examination on pleural fluid was positive in 8 (40%) cases, negative in 7 (35%) and not performed in 5 (25%) cases; pericardial histology was positive in 8 (40%) cases, negative in 7 (35%) and not performed in 5 (25%) cases.
Intraoperative mean volume of the drained pericardial effusion was 548.5 mL (range, 250–780 mL); mean duration of the procedure was 78.7 minutes (range, 38–126 minutes); postoperative mean length of stay was 4.4 days (range, 2–7 days); 5 (25%) patients had postoperative complications: 3 (15%) had atrial fibrillation (Minor, Grade I); 1 (5%) acute respiratory failure requiring ICU admission and non-invasive ventilation (Major, Grade IIIa); 1 (5%) had gastric bleeding and died within 30 days (Mortality, Grade V); 11 (55%) patients underwent concomitant procedures: bilateral talc poudrage in five cases, monolateral talc poudrage in five cases, supraclavicular Daniels biopsy in one case.
Eight patients (40%) had undergone previous surgical treatments for pericardial effusion: 4 received pericardiocentesis, 3 received pericardial drainage and 2 of them sclerosing intracavitary treatment with thiotepa; 1 patient received pericardiopleural window and left talc poudrage (Table 2).

Full table
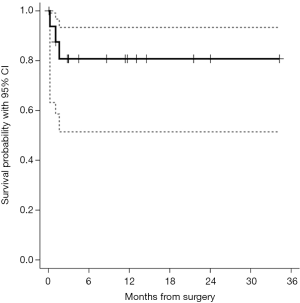
Median duration of follow-up was 11.4 months. After 34 months of follow-up the OS was 80.8% (51.4–93.3%) (Figure 3).
Discussion
Pericardial effusion is an increasingly common complication of neoplastic diseases and can be life-threatening not only in patients with terminal malignancies but also in those with a more favorable prognosis (9). In fact, the improved survival of cancer patients—strictly related to new biological and immunotherapeutic approaches—has led to an increased incidence of secondary patterns such as malignant pericardial effusion (9).
To be effective, the ideal procedure for treating pericardial effusion should not only relieve cardiac tamponade immediately but must also prevent recurrence. Pericardiocentesis alone is associated with a high recurrence rate (60–100%) even when repeated frequently (10), while pericardial drainage with injection of sclerosing agents may optimize pleural effusion treatment, but the success rate is variable (11). Surgical pericardial windowing through thoracotomic, thoracoscopic or subxiphoid approaches offer the best long-term results minimizing pericardial effusion recurrence and providing adequate specimens for cytohistological assessment (12-14).
Ten of the 20 patients in our cohort received concomitant monolateral or bilateral videothoracoscopic talc poudrage to treat synchronous pleural effusion. Although it may appear easier and faster to perform a pleuropericardial window during VATS talc poudrage, we suggest this approach be avoided because lung reexpansion and effective pleurodesis would finally lead to lung mediastinal adhesions thereby nullifying the effect of the pleuropericardial window. In fact, one of our patients was submitted to emergency pericardioperitoneal window due to effusion recurrence after failure of pleuropericardial window performed less than 30 days earlier. Moreover, the subdiaphragmatic recess in the pericardial-peritoneal window and the whole abdominal cavity act as a collection chamber where the fluid is gradually reabsorbed by the peritoneum, offering a large and more effective reabsorption surface than the pleural cavity (15).
Preoperative assessment is based on ultrasound and CT scan: echocardiographic evaluation allows a dynamic study of cardiac function, focusing on the evidence of diastolic right ventricle collapse which is commonly recognized as the most specific sign of cardiac tamponade (16), while CT scan evaluates concurrent pleural effusions (Figure 3). Although both methods were effective in terms of effusion estimate by quantification of the maximum detachment between pericardium and cardiac surface (17), echocardiography appears to be a more accurate imaging technique than CT in the quantitative assessment of non-loculated pericardial effusion and should continue to be the primary imaging modality in these patients (18).
In our case series the OS after 34 months of follow-up was 80.8%. Given the tumor heterogeneity of our study group (lung, breast, kidney, mesothelioma and ovarian cancers), this observation is not intended to offer any oncology forecast, but should be considered a benchmark for the appropriateness of surgical indications. As pericardioperitoneal window is always performed in metastatic patients, it should be considered only for patients with a good short-term prognosis and life expectancy, whereas pericardial drainage could be advocated as an adequate treatment in patients with a less favorable prognosis.
Eight patients were previously submitted to different treatments before undergoing pericardial peritoneal window, the most common being pericardiocentesis and pericardial drainage. On the one hand, this highlights how pericardioperitoneal window can be considered the most effective treatment in terms of effusion recurrence and may offer good results even after other techniques have failed. On the other, the procedure should not be performed in an emergency setting, when pericardiocentesis or pericardial drainage should be preferred (19,20).
In conclusion, pericardial-peritoneal window is a safe and effective procedure to resolve malignant pericardial effusion in patients with a favorable short–term prognosis, whereas pericardial drainage should be considered the most appropriate treatment in patients with a less favorable prognosis (21).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Azam S, Hoit BD. Treatment of pericardial disease. Cardiovasc Ther 2011;29:308-14. [Crossref] [PubMed]
- Wagner PL, Stillwell E, Bott M, et al. Pericardial effusions in the cancer population: prognostic factors after pericardial window and the impact of paradoxical hemodynamic instability. J Thorac Cardiovasc Surg 2011;141:34-8. [Crossref] [PubMed]
- Allen KB, Faber LP, Warren WH, et al. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg 1999;67:437-40. [Crossref] [PubMed]
- Larrey EL. New surgical procedure to open the pericardium in the case of fluid in the cavity. Clin Chir 1829;36:303-37.
- Miller JI, Mansour KA, Hatcher CR Jr. Pericardiectomy: current indications, concepts, and results in a university center. Ann Thorac Surg 1982;34:40-5. [Crossref] [PubMed]
- Hazelrigg SR, Mack MJ, Landreneau RJ, et al. Thoracoscopic pericardiectomy for effusive pericardial disease. Ann Thorac Surg 1993;56:792-5. [Crossref] [PubMed]
- Shapira OM, Aldea GS, Fonger JD, et al. Video-assisted thoracic surgical techniques in the diagnosis and management of pericardial effusion in patients with advanced lung cancer. Chest 1993;104:1262-3. [Crossref] [PubMed]
- Ivanovic J, Al-Hussaini A, Al-Shehab D, et al. Evaluating the reliability and reproducibility of the Ottawa Thoracic Morbidity and Mortality classification system. Ann Thorac Surg 2011;91:387-93. [Crossref] [PubMed]
- Martinoni A, Cipolla CM, Cardinale D, et al. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest 2004;126:1412-6. [Crossref] [PubMed]
- Sugimoto JT, Little AG, Ferguson MK, et al. Pericardial window: mechanisms of efficacy. Ann Thorac Surg 1990;50:442-5. [Crossref] [PubMed]
- Shepherd FA, Ginsberg JS, Evans WK, et al. Tetracycline sclerosis in the management of malignant pericardial effusion. J Clin Oncol 1985;3:1678-82. [Crossref] [PubMed]
- Ancalmo N, Ochsner JL. Pericardioperitoneal window. Ann Thorac Surg 1993;55:541-2. [Crossref] [PubMed]
- Piehler JM, Pluth JR, Schaff HV, et al. Surgical management of effusive pericardial disease. Influence of extent of pericardial resection on clinical course. J Thorac Cardiovasc Surg 1985;90:506-16. [PubMed]
- Dean RH, Killen DA, Daniel RA Jr, et al. Experience with pericardiectomy. Ann Thorac Surg 1973;15:378-85. [Crossref] [PubMed]
- Kallianpur AA, Samra SS, Nimbran V, et al. Pericardial-peritoneal window: a novel palliative treatment for malignant and recurrent cardiac tamponade. Indian J Palliat Care 2013;19:116-8. [Crossref] [PubMed]
- Tsang TS, Freeman WK, Sinak LJ, et al. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc 1998;73:647-52. [Crossref] [PubMed]
- Maggiolini S, De Carlini CC, Ferri LA, et al. The role of early contrast-enhanced chest computed tomography in the aetiological diagnosis of patients presenting with cardiac tamponade or large pericardial effusion. Eur Heart J Cardiovasc Imaging 2016;17:421-8. [Crossref] [PubMed]
- Leibowitz D, Perlman G, Planer D, et al. Quantification of Pericardial Effusions by Echocardiography and Computed Tomography. Am J Cardiol 2011;107:331-5. [Crossref] [PubMed]
- Maggiolini S, Gentile G, Farina A, et al. Efficacy, and Complications of Pericardiocentesis by Real-Time Echo-Monitored Procedure. Am J Cardiol 2016;117:1369-74. [Crossref] [PubMed]
- Seferović PM, Ristić AD, Imazio M, et al. Management strategies in pericardial emergencies. Herz 2006;31:891-900. [Crossref] [PubMed]
- Adler Y, Charron P, Imazio M, et al. European Society of Cardiology (ESC) 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64. [Crossref] [PubMed]
Cite this article as: Petrella F, Radice D, Colombo N, Mariolo AV, Diotti C, de Marinis F, Spaggiari L. Pericardial-peritoneal window for malignant pericardial effusion. Shanghai Chest 2018;2:45.