
Surgical technique of pleurectomy/decortication
Introduction
Malignant pleural mesothelioma (MPM) is an extremely aggressive thoracic malignancy with poor prognosis despite several therapeutic options (1,2). Two different approaches have been introduced as curative surgery options for MPM. Extrapleural pneumonectomy (EPP), which is the lung sacrificing surgery, involves an en bloc resection of the parietal pleura, ipsilateral lung, diaphragm, and pericardium. In contrast, pleurectomy/decortication (P/D), which is lung sparing surgery, involves the removal of only the parietal and visceral pleura while preserving the lung parenchyma (3-6). Unlikely other solid malignancies, neither EPP nor P/D allow for a suitable surgical margin. Indeed, both EPP and P/D are recognized as cytoreductive surgeries with the goal of macroscopic complete resection (MCR) (2,7). As there is no reliable evidence that demonstrates that curative intent surgery actually improves prognosis, it should be performed as a part of multimodal therapy in patients that can achieve MCR (8,9).
The choice of surgical approach, which is supported only by limited reliable evidence, remains controversial (4,7,10). Even though EPP has been the first choice of curative surgery over the past decades, the preferable approach has now shifted to P/D. This may be because of the fact that EPP is an extremely invasive approach that is associated with high mortality and morbidity rates. Furthermore, Rena et al. have reported that the postoperative quality of life was extremely poor owing to not only its invasiveness but also the poor status of cardiopulmonary function based on pneumonectomy (11). However, P/D is also an invasive surgery with a complicated surgical procedure. Herein we describe this surgical procedure and some of the problems of P/D.
Indications for P/D
The indications of curative intent surgery for MPM are as follows:
- Histologically proven MPM;
- Patients that are expected to obtain MCR;
- Feasible to perform multimodal therapy;
- Tolerable for the curative intent surgery.
Furthermore, Rusch et al. has described that therapeutic strategy using the multimodal therapy should be performed by a multidisciplinary team including surgeons, medical oncologists, radiation oncologists, and pathologists (7). Tolerance for surgical treatment is also essential owing to the invasive nature of the surgery, and should be evaluated thoroughly. In general, a good performance status (PS) and sufficient cardiopulmonary function are needed for candidates who undergo EPP and P/D.
In the Hyogo College of Medicine, the surgical criteria for curative intent surgery are as follows:
- Radiologically resectable (c-T0-3);
- Excluded histologically proven sarcomatoid type;
- Excluded proven N2.
Moreover, a strict evaluation of tolerance for the surgical treatment in cases of EPP followed these guidelines:
- Sufficient cardiopulmonary function;
- Predicted residual FEV1.0 >1,000 mL;
- Age <75 years old.
In contrast, the indications of surgical treatment for P/D is comprehensively determined on the basis of cardiopulmonary function and tumor status.
Definition of P/D
Previously, the definition of P/D was not clarified. This is probably because P/D has been performed not only curative intent surgery but also palliative surgery for MPM, and there were some variations among each surgeon with regard to procedure including extent resection with surrounding organs.
In 2011, International Association of Society of Lung Cancer (IASLC) and International of Mesothelioma Interest Group (IMIG) described the consensus report of the definition of P/D (12).
Extended P/D
Parietal and visceral pleurectomy to remove all gross tumor with resection of the diaphragm and/or pericardium. The IASLC Mesothelioma Domain suggests use of the term “extended” rather than “radical” in this instance as the latter implies a completeness of resection with added therapeutic benefit. There is currently insufficient evidence that resection of the pericardium and diaphragm provides either.
P/D
Parietal and visceral pleurectomy to remove all gross tumor without resection of diaphragm and pericardium.
Partial pleurectomy
Partial removal of parietal and/or visceral pleura for diagnostic or palliative purposes but leaving gross tumor behind.
By the description of this report, only extended P/D and P/D meet the curative intent surgery criteria for MPM. However, the difference between R2 resection and exploratory thoracotomy were still unclear and the determination would be left to each surgeon.
Helpful technique or equipment for P/D
Blunt dissection using cotton gloves (Figure 1)
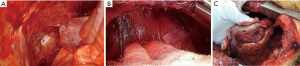
Unlike common thoracic surgery, a bunt approach is very helpful in P/D (13,14) as it provides the surgeon the following several benefits. A blunt approach makes the extrapleural dissection easy and prompt. Surgery for MPM, especially P/D, involves huge planes of the chest wall and ipsilateral lung. It takes a great amount of time using a sharp approach with scissors or electrocautery like other general thoracic surgical procedures. In contrast, blunt dissection enables the surgeon to cover a wide area quickly. Blunt dissection can also lead to an appropriate dissection plane. It is based on the detachment of loose layers, but in cases where tumor has infiltrated the lung parenchyma or chest wall, the technique becomes more difficult. Thus, blunt dissection helps to find tumor infiltration during surgery. Conversely, sharp dissection can dissect regardless of tumor infiltration, which may lead incorrect dissection plane. We prefer to use cotton gloves and swabs for blunt dissection. Cotton gloves help to hold the pleura strongly and gently and are helpful during the dissection in early stage MPM with normal-like pleura.
Monitor vision (Figure 2)
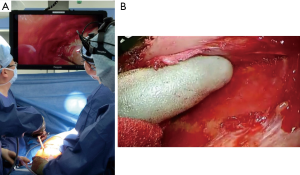
As P/D is still a highly invasive procedure, an effort must be made to reduce its invasiveness. We consider video-assisted thoracic surgery (VATS) as one of the possible solutions, and routinely use VATS in surgeries for MPM. VATS can not only provide favorable surgical views of deep lesions, but also allows for the recognition of an appropriate layer for dissection. With a favorable surgical view through the VATS monitor, we can perform a dissection of the apical or upper mediastinum area. As a result, our thoracotomy shifted to lower intercostal spaces, and neither dividing anterior costal arch nor additional thoracotomies were needed. This current approach can also preserve chest wall rigidity and reduce surgical invasiveness. Moreover, VATS monitors provide clear sight of enlarged objects and contributes to a precise dissection to preserve the surrounding organs, such as the pericardium and diaphragm. Indeed, the number of patients that need reconstruction of the pericardium or diaphragm through this current approach significantly decreased at our institution.
VIO soft-coagulation system (Figure 3)
We sometimes experience continuous bleeding on dissected planes during the dissection of parietal pleura, especially in case of concomitant inflammation. We also experience bleeding caused by lung parenchyma injury during decortication. As P/D takes a long time, the appropriate control of any bleeding is essential. We prefer to use the VIO soft-coagulation system (ERBE Elektromedizin, Tubingen, Germany) in which the output voltage is automatically regulated (15,16). The VIO system, which is autoregulated to an output voltage below 200 V, can be helpful to control the bleeding by using a temperature below the boiling point without generating sparks. As the VIO system works only on the surface of attached tissue without causing damage to surrounding organs, it is very efficient in controlling the bleeding from lung parenchyma during decortication. Furthermore, the edge of the VIO system is shaped as a ball, which is applicable to not only lung parenchyma but also the whole dissection plane.
Surgical procedure
The surgical procedures of P/D comprise two parts. The first half (step 1) is from the thoracotomy to the end of the extrapleural dissection, which is similar to EPP. The second half (step 2) is from the dissection of the visceral pleura until the chest is closed.
Step 1-1: thoracotomy
A simple posterolateral thoracotomy at the 7th rib bed is employed. En bloc resection of previous port sites with skin and full thickness chest wall are required. Neither the division of anterior costal arch nor additional thoracotomies are required. This thoracotomy offers direct visualization of the whole diaphragm and pericardium. Conversely, a thoracoscopic monitor is required for the manipulation of apical areas.
Step 1-2: extrapleural dissection of the lateral wall
Extrapleural dissection is started at the thoracotomy site and extended to the apical, mediastinal, pericardial, and diaphragmatic regions. Usually, extrapleural dissection in the lateral area is not difficult and can be performed quickly by blunt dissection using cotton gloves or swabs. Both tumor invasion and inflammatory adhesions are possible in cases of difficult dissections, and are sometimes indistinguishable; confirmation by frozen section is required in such cases. Combined chest wall resection can be considered if the tumor invasion is limited. When diffuse chest wall invasion of the tumor is recognized, the operation should be abandoned.
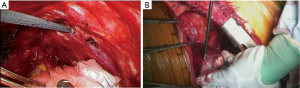
Step 1-3: extrapleural dissection at the apex (Figure 4A)
Extrapleural dissection of the apical pleura is generally simple to perform. However, dissection may be very difficult and dangerous when the parietal pleura is hardly fixed to the apical chest wall. Because this is the deepest area of the thoracotomy, aggressive blunt dissection in a blind fashion could lead to catastrophic bleeding from subclavian vessels.
Step 1-4: extrapleural dissection at the mediastinum (Figure 4B-D)
Recognition of the dissection plane between the parietal pleura and the mediastinal organs is very important. Usually, the parietal pleura is easily dissected from the mediastinum. Otherwise, possible extrapleural tumor invasion should be considered. Tumor sometimes involves the junctions of the superior vena cava/azygos vein and the azygos vein/intercostal veins in the right hemithorax, and the junction of the aortic arch/descending aorta in the left hemithorax. Care should be taken not to damage the phrenic, vagal, recurrent laryngeal, and sympathetic nerves.
En bloc blunt dissection of the pericardium and the pericardial fat pad is usually successful. Pericardial resection is performed only when tumor invasion is recognized. Irrespective of the pericardial resection, pericardial effusion cytology is recommended to evaluate the tumor invasion. We abandon EPP in cases of positive pericardial effusion cytology.
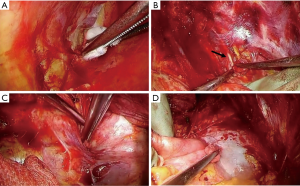
Step 1-5: extrapleural dissection at the diaphragm (Figure 5)
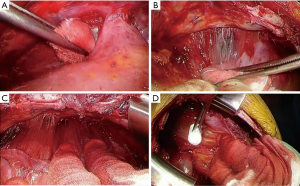
The dissection of the diaphragm is the most difficult part of the surgery for MPM (14). This is partly because of the tight connections of the parietal pleura and the diaphragm and poor surgical vision; precise dissection in this area requires technical skill and patience. Some authors resect the diaphragm to confirm complete resection (17). We prefer to make maximal efforts to preserve the diaphragm as far as possible. This is why we perform the thoracotomy at the 7th intercostal bed instead of the 5th or 6th. Avoiding patch reconstruction could enhance postoperative adhesion between the diaphragmatic muscle and the decorticated lung, reduce air leakage, and reduce intrathoracic infection. Regarding oncological aspects, this contributes to the avoidance of tumor seeding into the peritoneum.
Blunt dissection is performed by peeling off the diaphragm from the parietal pleura while holding the pleural sac with cotton gloves and using a swab. If the resection of the diaphragm is inevitable because of tumor invasion or tight adhesion, efforts should be made to preserve sufficient mediastinal margins of the diaphragm for suturing artificial patches. Postoperative patch dehiscence mostly occurs in this area. In case of peritoneal injury, immediate repair is recommended to avoid tumor dissemination.
Step 2-1: visceral pleurectomy (Figure 6)
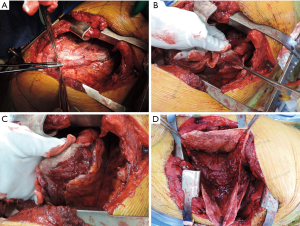
Visceral pleurectomy is usually started at the lateral segment (segment 8) of the lower lobe, which consists of a wide flat plane. Firstly, we make a 5–10 cm long incision in the visceral pleura using scissors or a blade. Further, the cut end of the visceral pleura is gripped with cotton gloves and peeled off from the lung parenchyma. Occasionally, the parietal pleura is spontaneously dissected. Otherwise, very gentle blunt dissection using dry gauze or swabs are recommended. Dissection of the interlobar vessels is usually easy. Dissection of the visceral pleura at the apex and base is often difficult, and sometimes requires sharp dissection. The above procedure is far easier in cases with complete pleural adhesions. This is why we routinely perform pleurodesis with talc after thoracoscopic pleural biopsy. Partial pulmonary resection or lobectomy is acceptable when residual tumor on the pulmonary parenchyma is suspicious. Although some surgeons prefer to perform visceral pleura dissection for inflated lungs (14), we dissect the visceral pleura with collapsed lung. The preference may depend on the surgical technique employed during the visceral pleurectomy.
Step 2-2: reconstruction of the diaphragm and pericardium
In cases of minor resection of the diaphragm and pericardium, direct suture is preferable. Otherwise, the pericardium is reconstructed with a 0.1 mm Gore-Tex patch (PRECLUDE® Pericardial membrane 1PCM102; W.L. Gore & Associates, Inc., Arizona, USA), and the diaphragm is reconstructed with a 2 mm Gore-Tex patch (GORE-TEX® Soft Tissue Patch 13150S; W.L. Gore & Associates, Inc., Arizona, USA). Pericardial patches should be loosely fixed to prevent postoperative restrictive cardiac disorders, including cardiac tamponade. Unlike EPP, the attachment of diaphragm patches is sometimes more cranial than the anatomical position in P/D, in order to reduce residual thoracic space, facilitate adhesions, and shorten the duration of air leakage (18).
Step 2-3: repair of lung parenchyma (Figure 7)
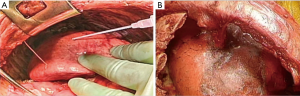
Air leakage because of visceral pleurectomy is inevitable in P/D. Massive air leakage from visible airways must be sutured. Fibrin glue is routinely used to control air leakage from lung parenchyma. Bioabsorbable sheets are also applicable and effective in controlling air leakage, but they often interfere with lung expansion.
Step 2-4: closure
We usually use three drainage tubes after P/D. Two 24-French straight drainage tubes are placed to the apex (one at the anterior side, and another posterior). One L-shaped 24-French drainage tube is placed on the diaphragm. These drainage tubes are suctioned at −5 cm H2O.
Conversion to EPP
Conversion from P/D to EPP is decided in cases where intraoperative findings indicate that MCR can only be achieved by EPP because of diffuse residual tumor on the pulmonary parenchyma. This situation can occur even in patients with early disease. Therefore, preoperative pulmonary function test and ventilation/perfusion scans should be performed in all surgical candidates. Preoperative discussions with patients on whether or not they accept conversion is also important.
Controversies of P/D
Intracavitary treatment
It is well known that the most frequent failure pattern after multimodality therapy including P/D is local recurrence (19). Several additional local treatments have been employed to avoid postoperative local recurrence. The efficacy of hyperthermic intraoperative pleural chemotherapy (HIPC) has been reported in MPM and other malignancies, such as peritoneal mesothelioma and ovarian cancer. Sugarbaker et al. described how HIPC contributed to not only local recurrence control, but also improved overall survival (20). Opitz et al. reported that fibrin carried cisplatin (CDDP) therapy could deliver higher concentration of CDDP to the targeted area with less toxicity (21). Phase II clinical trial of fibrin carried CDDP therapy has been performed (2). Friedberg et al. reported how intracavitary photodynamic therapy (PDT) yields the longest disease-free survival and overall survival (22). Although the efficacy of intracavitary therapy is suggested in this way, it has not been standardized at the present time.
Early stage MPM without gross tumor
As previously described, MCR is the goal in the surgical treatment for MPM. However, we sometimes confuse the definition of MCR when we encounter very early stage MPM with no macroscopic tumor on both pleura (23). Do we need to resect 100% of the pleura, including areas without macroscopic lesions, to achieve MCR? Does the employment of laser ablation instead of pleural resection meet the definition of MCR (24)?
Conclusions
Here we described the current status of P/D, and also our current practice with it. However, unlike in EPP, there still remains considerable technical variations in P/D. Efforts should be made to standardize the surgical technique.
Although P/D is less invasive than EPP, a further reduction in surgical insult is required for better postoperative quality of life.
Acknowledgements
We would like to thank Miss. Risa Murata for secretarial work, and Enago (www.enago.jp) for the English language review.
Footnote
Conflicts of Interest: S Hasegawa received honoraria from Eli Lilly Company and Taiho Pharmaceutical Company. M Hashimoto has no conflicts of interest to declare.
References
- Hasegawa S, Tanaka F. Malignant mesothelioma: current status and perspective in Japan and the world. Gen Thorac Cardiovasc Surg 2008;56:317-23. [Crossref] [PubMed]
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Takuwa T, Hasegawa S. Current surgical strategies for malignant pleural mesothelioma. Surg Today 2016;46:887-94. [Crossref] [PubMed]
- Hasegawa S. Extrapleural pneumonectomy or pleurectomy/decortication for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2014;62:516-21. [Crossref] [PubMed]
- Rice D. Standardizing surgical treatment in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:497-501. [PubMed]
- Rusch VW. Extrapleural pneumonectomy and extended pleurectomy/decortication for malignant pleural mesothelioma: the Memorial Sloan-Kettering Cancer Center approach. Ann Cardiothorac Surg 2012;1:523-31. [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Mesothelioma Weder W. Ann Oncol 2010;21 Suppl 7:vii326-33. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Weder W, Opitz I. Multimodality therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:502-7. [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung cancer 2012;77:151-5. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Friedberg JS. The state of the art in the technical performance of lung-sparing operations for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2013;25:125-43. [Crossref] [PubMed]
- Sakuragi T, Ohma H, Ohteki H. Efficacy of SOFT COAG for intraoperative bleeding in thoracic surgery. Interact Cardiovasc Thorac Surg 2009;9:767-8. [Crossref] [PubMed]
- Uchiyama A, Miyoshi K, Nakamura K. VIO soft-coagulation system for major pulmonary resections: results in 68 patients with primary lung cancer. Gen Thorac Cardiovasc Surg 2011;59:175-8. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Schirren J. Thoracic shaping technique to avoid residual space after extended pleurectomy/decortication. Eur J Cardiothorac Surg 2013;44:563-4. [Crossref] [PubMed]
- Kostron A, Friess M, Crameri O, et al. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1516-23. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Opitz I, Erne BV, Demirbas S, et al. Optimized intrapleural cisplatin chemotherapy with a fibrin carrier after extrapleural pneumonectomy: a preclinical study. J Thorac Cardiovasc Surg 2011;141:65-71. [Crossref] [PubMed]
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65;discussion 1665-57.
- Hasegawa S, Kondo N, Matsumoto S, et al. Practical approaches to diagnose and treat for T0 malignant pleural mesothelioma: a proposal for diagnostic total parietal pleurectomy. Int J Clin Oncol 2012;17:33-9. [Crossref] [PubMed]
- Bölükbas S, Biancosino C, Redwan B, et al. Diode-Pumped Laser for Lung-Sparing Surgical Treatment of Malignant Pleural Mesothelioma. Ann Thorac Surg 2017;103:e529-30. [Crossref] [PubMed]
Cite this article as: Hashimoto M, Hasegawa S. Surgical technique of pleurectomy/decortication. Shanghai Chest 2018;2:44.


