VATS pneumonectomy and sleeve carinal resections—full thoracoscopic and uniportal approach
In recent years endoscopic technology has started to play an important role in surgical treatment of thoracic and abdominal tumors. Many surgeons have applied the so-called Golden Standard for surgical treatment of a number of malignant neoplasms using endoscopic technology. Endoscopic technology is widely used in treatment in a variety of oncological procedures such as colectomy, nephrectomy, total hysterectomy, distal gastrectomy, thoracoscopic major pulmonary resections and in many other surgical interventions. Being successfully developed and introduced into clinical practice, more advanced surgical procedures with labour-intensive reconstructive steps are performed by several surgeons in a small number of hospitals. These kinds of surgical procedures seem difficult to replicate due to technical problems and the length of learning curve. In some cases whether or not to opt for the endoscopic access is determined either by the complexity and safety of the reconstructive step (pancreatoduodenectomy as an example) or by the necessity to make a rather big incision for removing a specimen (laparoscopic right hepatectomy, large retroperitoneal or mediastinal tumors and thoracoscopic pneumonectomies). Nevertheless, these surgical procedures are feasible for a small group of patients and are performed with good outcomes both in the immediate and long-term results.
Advantages of thoracoscopic pulmonary resections can’t be doubted and are confirmed by numerous studies (1-6). These procedures allow significantly reducing surgical trauma and blood loss, decreasing the length of hospital staying and less the need for anesthetics. Other benefits include early recovery, rapid rehabilitation and improvement of quality of life. Moreover, if needed, adjuvant chemotherapy might be provided earlier.
Despite the high incidence rates of lung cancer in the world and the fact, which most patients present with stage II or, predominantly, stage III of the disease, there has been a decrease in the number of pneumonectomies over the past years. It is attributed to novel neoadjuvant schemes of treatment, which has recently come into play, as well as to the emphasis on organ sparing surgeries with angioplasty and bronchoplasty. However, for some patients with centrally located lung tumors or with tumor infiltrating of hilar structures or interlobar fissure, the only possible surgical procedure is radical pneumonectomy. One of the major problems in selecting patients for thoracoscopic pneumonectomy is unavailability of palpation and lack of full intraoperative revision in order to evaluate tumor spreading. Both to assess the tumor spreading using palpation and to remove large specimens, Detterbeck and colleagues have suggested using substernal approach, which allows inserting a surgeon’s hand into the chest (7).
First left thoracoscopic pneumonectomy was performed by Walker in 1993. Pulmonary artery and veins were cut using a linear stapler with forceps placed beforehand on the main trunks to control possible bleeding. The lung was removed via minithoracotomy in the V intercostal space. The patient was discharged on the 5th hospital day without any complications and with lower pain syndrome compared to surgeries using posterolateral thoracotomy (8). In the following years there have been a lot of publications dedicated to a constantly growing number of patients who underwent thoracoscopic surgery (major pulmonary resections) (9-11). There were not many reports about pneumonectomies performed with the thoracoscopic approach and they didn’t go beyond a description of few small series or case reports. Meanwhile, some authors admitted to using up to 16-cm long, which allowed to use conventional surgical instruments and to provide direct visual control of the hilar structures (12,13). Accordingly, there were only 6 minimally invasive video-assisted pneumonectomies performed over a period of 2 years in Walkers’s hospital, (2 on the right and 4 on the left). In the authors’ opinion, patients after pneumonectomy made a much better postoperative recovery in contrast with those who underwent thoracoscopic lobectomy owing to the absence of air leakage-associated complications. Major postoperative problems after pneumonectomy were related to cardiac rhythm disturbances (14). Some authors give a detailed description of radical pneumonectomy using thoracoscopic approach (15-17). Conlan et al. gave a description of the left thoracoscopic pneumonectomy performed via three incisions of 2 cm without minithoracotomy. Given the difficulty of the main left bronchus separation from the aortal window, the latter was transected in the distal part, and then the lung was removed. After that the bronchial stump was resected using linear stapler and the suture line was reinforced with Prolene 2/0.
Upon gaining more experience in thoracoscopic interventions, a number of video-assisted pneumonectomies haven’t increased significantly. In 2006, Demmy et al. reported about 7 thoracoscopic pneumonectomies out of 25, performed over 2 years. Central tumors less than 5 cm in size, infiltration of the central part of the fissure, impossibility to perform any broncho-angioplasty procedure and synchronous ipsilateral tumors served as primary indications for radical pneumonectomy. The procedure included the mediastinal sampling or lymphadenectomy. In 4 years, the same group of authors published the results of treatment for another 24 patients, who had undergone thoracoscopic pneumonectomy with 25% of conversion. Benefits of thoracoscopic surgeries were related only to the length of stay and the amount of blood loss, which both appeared to be significantly lower. In terms of survival rates, the authors have not yet come to a well-defined conclusion, pointing out that they are not worse than those in open surgeries. In addition to lung cancer surgeries, there have been only few mentions of using thoracoscopic pneumonectomy in treating emphysema and pulmonary histoplasmosis (18-21).
In 2014, Kim and co-authors reported the results of seven surgeries, performed over 4 years. The average operating time was 6 hours (from 4 to 12 hours). The longest time required those patients who had previously undergone surgery due to the presence of pleural adhesions. The amount of blood loss varied from 150 to 700 mL. Two patients developed the bronchial stump fistula during the first postoperative week and in 4 months. The authors attribute relatively large number of complications to the time of acquiring the method and to a small quantity of patients (22).
In spite of the overall implementation of thoracoscopic technologies in lung cancer treatment (lobectomy and segmentectomy), there is a relatively small number of hospitals exercising a thoracoscopic approach for pneumonectomy (23-27). Nevertheless, some clinics aim at decreasing surgical trauma during traumatic surgeries by looking for new minimally invasive techniques, such as robotic and single-port pneumonectomy, including carinal resection (28-31). Although a single-port approach for pneumonectomy is rather difficult to execute, highly experienced thoracic surgeons perform these procedures with satisfactory primary and long-term outcomes (32,33).
Bearing in mind that oncological principles in thoracoscopic surgery are similar to those in open one, in this article we are going to dwell in important technical details of thoracoscopic pneumonectomy, performed by full thoracoscopic and uniportal approach.
A special kit of thoracoscopic instruments is used to perform thoracoscopic major pulmonary resections, as well as full HD, 4K or 3D video system, staplers and clippers (Figure 1).
The patient is placed to the lateral decubitus position. In the projection of the V intercostal space, a roller is raised by 5–10 cm and the angle of operating table is approximately 150 degrees. In case of uniportal approach a surgeon is always facing a patient (Figure 2A).

During totally endoscopic left pneumonectomy a surgeon also stands in front of a patient, while during the right one behind him (Figure 2B). A necessary condition is that a patient is immobilized with safety straps or air matrass for ensure safe tilts of an operating table.
Taking into account a necessity to extract large specimen, we have modified the position of the thoracic ports. To dissect and divide hilar structures, and also to get the visual imaging of the operating field, three ports located in the central part of the hemithorax are used.
The biggest 30 mm port is made in the X intercostal space near anterior border of latissimus dorsi muscle. Via this port aorta and esophagus contraction is done, along with the insertion of the endoscopic stapler to dissect vascular structures and the main bronchus. Fluctuant X rib does not have a fixation to the arcus rib, which makes it possible to enlarge this port by dissecting the muscle layer-by-layer and to extract quite a big specimen in container via a 5–6 cm incision. The advantages of this approach are absence of pressure on the intercostal nerve, good functional and cosmetic outcome. The main steps of any radical surgery for lung cancer (segmentectomy, lobectomy or pneumonectomy) are: resection (removal of lung or its part) and lymphadenectomy, which might be performed by sampling or systematic ipsilateral mediastinal lymphadenectomy. Taking into account high probability of lymph node metastasis of lung cancer, we consider the latter option to be worthwhile as it allows performing a radical surgery and provide adequate tumor staging. That is why we prefer to begin a surgery with systematic ipsilateral mediastinal lymphadenectomy with frozen section, if it necessary—“Nodes first” technique. Having performed lymphadenectomy first, we can then decide on the extent of lung parenchyma resection (in case of segmentectomy or lobectomy), achieve complete lung collapse, isolate and prepare all anatomical structures for dividing.
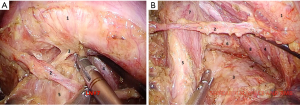
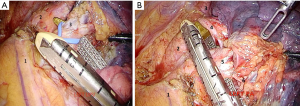
In case of totally thoracoscopic approach the first port (optical) is made in the V intercostal space along the anterior axillary line. After the thoracoscopic revision of pleural cavity optimal positions of the rest ports are marked according to the scheme. Then, an assistant performs lung traction upwards. Pulmonary ligament and mediastinal pleura are opened anterior and posterior to the hilum. Anterior mediastinotomy is performed up to the level of the arterial ligament along the phrenic nerve with removal lymph nodes between pulmonary veins. Posterior wall of the superior pulmonary vein is separated from the upper lobe bronchus and taken on vascular tape (Figure 3A,B).

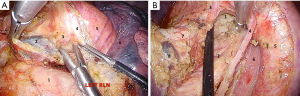
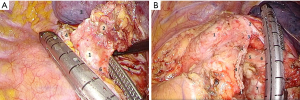
After that we proceed with the posterior hilar and subcarinal lymphadenectomy for which endo-retractors seem worth using because they provide good access to the posterior mediastinum due to traction of left lung anteriorly. Retractor sutures could be extracted via trocar holes, as well as through a puncture of chest wall by a thin needle. Hook and harmonic scalpel are used to transect the mediastinal pleura covering aorta and the posterior surface of the hilum. Mid thoracic esophagus is identified along and separated from the posterior wall of the pericardium and from block of #7 lymph nodes to the opposite pleural sac and the membranous part of the trachea. At the same time left bronchial arteries raised from thoracic aorta are being clipped and cut (Figure 4A). After that an assistant performs traction of esophagus upward and backward, thus giving space for the dissection lymph nodes. Then we dissect low edge of left main bronchus, posterior wall of the pericardium, medial wall of right main bronchus and carina. At this stage it is necessary to prevent contact the membranous part of the trachea and main bronchi with an active blade of a harmonic scalpel. The final view of the subcarinal zone is showed in Figure 4B.
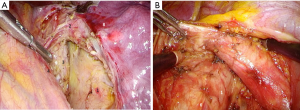
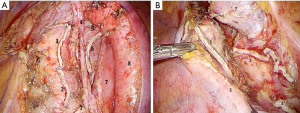
To ensure maximum traction of left main bronchus for making a short bronchial stump, it is important to mobilize esophagus from the membranous part up to the level of aortic arch. Tracheobronchial and subaortic lymph nodes are removed at the same time. At this stage cold scissors and a dissector are best options for the dissection in order to minimize the risk of left recurrent laryngeal nerve damage (Figure 5A,B).
In case of right pneumonectomy, mediastinal lymphadenectomy includes hilar (#10), subcarinal (#7) and right paratracheal stations of lymph nodes (#2R and #4R). It should be mentioned that when removing a block of nodes, it is important to preserve blood supply of the tracheal wall and main bronchi for optimal conditions for healing bronchial stump or tracheobronchial anastomosis (Figure 6A,B).
To provide access to the main trunk of left pulmonary artery we consider starting with the dissection of upper pulmonary vein. After that it is save to dissect and staple the artery (Figure 7).
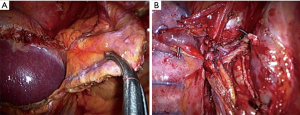
Lower pulmonary vein is the last to be cut. Mediastinal lymphadenectomy performed at the first stage of the surgery allows getting free access to the mobilized main bronchus. In order to provide stumpless technique on left main bronchus it is necessary to completely mobilize main bronchi and trachea bifurcation. Maximal traction is ensured by the endoscopic clamp or tape. Depending on the approach, the stapler is inserted through the X intercostal space or the anterior port, flexing so that its cartridge axis should be parallel to the axis of the opposite main bronchus. Stapler’s jaws should be placed in the anterior-posterior direction. An important technical point is using electromechanical stapler to provide smooth sewing of the bronchus wall. It is preferable to use cartridge with maximal height of the surgical clip and with the system ensuring smooth compression on the suture line (Figure 8). Final view of the pulmonary hilum and mediastinum after thoracoscopic pneumonectomy is shown in Figure 9.
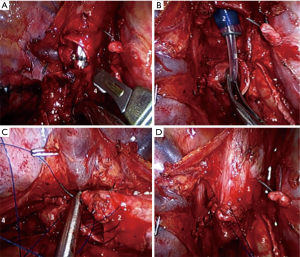
If the tumor spreads to the main bronchus involving the carina or tracheobronchial angle, it is necessary to perform wedge or sleeve carinal resection of the trachea bifurcation with direct anastomosis of the trachea and main bronchus. After the dividing of all vascular structures and mediastinal lymphadenectomy, trachea and left bronchus is transected with a scalpel and sharp scissors. Margins of trachea and main bronchus are sent to frozen section. If margins are negative we begin to form an anastomosis. High-frequency ventilation is ensured by a catheter placed in trachea through the left lumen of the double-lumen endotracheal tube (Figure 10A,B). Principles of the formation of thoracoscopic tracheobronchial anastomosis do not differ from those in open surgeries. Nevertheless, without sufficient experience this step can present considerable difficulties. We use monofilament sutures by V-LocTM (Medtronic, North Haven, CT, USA) or Prolene 3/0. In most cases we make a continuous suture on membranous and cartilaginous parts of the trachea and main left bronchus. The first bite is made into a left wall of the trachea and left main bronchus, after that, using a continuous suture; we sew the membranous and cartilaginous parts in such a way as to level the difference in diameter of the trachea and the main bronchus. The sutures are tied in the projection of the right angle of the anastomosis (Figure 10C,D).
After the anastomosis is completed, a water bubble test is taken for leaks. In all cases we try to cover the anastomosis by the mediastinal or diaphragmatic flap. From our point of view, the diaphragmatic flap is more reliable, but its dissection requires more time. With a harmonic scalpel the flap is formed from the medial part of the diaphragm. The blood flow is preserved due to the diaphragmatic vessels. The flap is covered and fixed by separate sutures to the line of the tracheobronchial anastomosis. Defect of the diaphragm is sewn using a continuous V-loc (Figure 11).
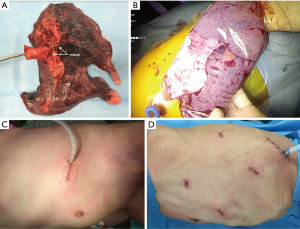
The specimen is extracted in a protective container though a single port or through the extended to 5 cm port in the X intercostal space. The pleural cavity is drained by one drainage (Figure 12).
Conclusions
Thoracoscopic access has many great advantages; it is relatively saved, much less traumatic and provides good imaging. Experience in endoscopic surgery allows significantly expanding the indications for thoracoscopic procedures. At the same time there is a tendency towards lung sparing surgery, reducing the number of pneumonectomies, which imposes limitations on performing minimally invasive procedures in this group of patients. Today, the main indication for thoracoscopic procedure is stage I–II lung cancer, when it is possible to perform segmentectomy or lobectomy. Nevertheless, there is a small group of patients for whom broncho-angioplasty is not possible and who require radical lung removal often with the resection of the surrounding structures and sleeve carinal resections. Taking into account problems with intraoperative revision patients should be thoroughly selected for pneumonectomy. At the same time, small number of reports about thoracoscopic pneumonectomy in literature show acceptable complications rate. Considering the same lymphadenectomy in open and endoscopic surgery we should expect similar long-term results, which should be analyzed in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.12). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1.100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg 2015;39:1084-91. [Crossref] [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- Detterbeck FC, Egan TM. Thoracoscopy using a substernal handport for palpation. Ann Thorac Surg 2004;78:1031-6. [Crossref] [PubMed]
- Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2. [Crossref] [PubMed]
- McKenna RJ Jr, Fischel RJ, Wolf R, et al. Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998;10:321-5. [Crossref] [PubMed]
- Saito A, Yagi N, Miura K, et al. Video-assisted right lower lobectomy for a lung cancer with mini-thoracotomy. Surg Laparosc Endosc 1995;5:56-8. [PubMed]
- Liu HP, Chang CH, Lin PJ. at al. Thoracoscopic assisted lobectomy. Preliminary experience and results. Chest 1995;107:853-5. [Crossref] [PubMed]
- Podbielski FJ, Marquez GD, Nelson DG, et al. Thoracoscopic assisted pneumonectomy. JSLS 1997;1:75-7. [PubMed]
- Yim AP. VATS major pulmonary resection revisited controversies, techniques, and results. Ann Thorac Surg 2002;74:615-23. [Crossref] [PubMed]
- Craig SR, Walker WS. Initial experience of video assisted thoracoscopic pneumonectomy. Thorax 1995;50:392-5. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C. Techniques of pneumonectomy: video-assisted thoracic surgery pneumonectomy. Chest Surg Clin N Am 1999;9:419-36. xi-xii. [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002;73:900-4. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Hung WT, Liao HC, Cheng YJ, et al. Nonintubated Thoracoscopic Pneumonectomy for Bullous Emphysema. Ann Thorac Surg 2016;102:e353-5. [Crossref] [PubMed]
- Kara HV, Javidfar J, Hirji SA, et al. Thoracoscopic pneumonectomy in management of histoplasmosis and fibrosing mediastinitis. Ann Thorac Surg 2014;98:e95-6. [Crossref] [PubMed]
- Kim AW, Fonseca AL, Boffa DJ, et al. Experience with thoracoscopic pneumonectomies at a single institution. Innovations (Phila) 2014;9:82-6; discussion 86. [Crossref] [PubMed]
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92. [Crossref] [PubMed]
- Wan IY, Thung KH, Hsin MK, et al. Video-assisted thoracic surgery major lung resection can be safely taught to trainees. Ann Thorac Surg 2008;85:416-9. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 1753.
- Spaggiari L, Galetta D. Pneumonectomy for lung cancer: a further step in minimally invasive surgery. Ann Thorac Surg 2011;91:e45-7. [Crossref] [PubMed]
- Louie BE. Robotic pneumonectomy. Thorac Surg Clin 2014;24:169-75. vi. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5:S246-52. [PubMed]
- Sekhniaidze D, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic sleeve resection. Ann Cardiothorac Surg 2016;5:145-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery: Pushing the Envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: a single centre experience. J Thorac Dis 2016;8:S235-41. [PubMed]
Cite this article as: Kononets P, Sekhniaidze D. VATS pneumonectomy and sleeve carinal resections—full thoracoscopic and uniportal approach. Shanghai Chest 2018;2:38.