Tunneled pleural catheters for management of malignant pleural effusions: a 2-year review of outcomes at a high-volume center
Introduction
Malignant pleural effusions (MPE) resulting from metastatic cancer represent a source of significant morbidity. MPE may arise from direct extension of tumor from the visceral pleura along with hematogenous or lymphangitic spread (1). The most common cancer associated with MPEs in men is lung cancer, while breast cancer represents the most common cause in women. Approximately 5–10% of patients with MPEs do not have a primary tumor identified (2,3). Median length of survival in patients with MPEs from presentation to death, ranges from 3–12 months and can be dependent on the type of underlying cancer (4).
Patients with MPEs often suffer significant shortness of breath due to impairment of diaphragmatic function and excursion (5). A number of therapeutic treatment options exist for patients suffering from MPE including serial thoracenteses, tunneled pleural catheter (TPC) placement, catheter directed pleurodesis, and surgical pleurodesis (6). Treatment algorithms are dependent on patient functionality, assessed by the Karnofsky score, pleural space physiology, and patient preference.
The use of TPCs for the treatment of MPE improves shortness of breath by improving diaphragmatic excursion and can result in scarring of the visceral and parietal pleura, known as autopleurodesis. Scarring obliterates the pleural space and prevents reaccumulation of fluid. TPCs can be efficiently placed in the outpatient setting, utilizing sedation or local anesthesia, and can improve patient quality of life. Autopleurodesis is generally accepted as 47–51% in the first 6 weeks after insertion (7,8). The literature reports an average of 6–14 weeks from catheter placement to autopleurodesis, with a complication rate of approximately 7% (8,9). Previous studies published reviewing the outcomes of TPCs describe relatively low volumes over prolonged periods of time (4). The present pilot data describes the overall patient experience, outcomes, and complications associated with the placement of TPC in MPEs over a 2-year period at a high-volume community hospital.
Methods
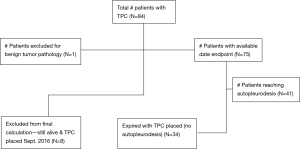
This retrospective pilot project describes a single institution’s clinical outcomes and complications in patients who received TPCs. The project aimed to be descriptive in nature and generate potential hypotheses for future study. Data was collected from electronic medical records of patients that had TPCs placed between September 2014 and August 2016 for MPEs. Inclusion criteria into analysis included patients aged 18 years and older, with an underlying malignancy, evidence of MPE, without having undergone previous talc pleurodesis, or any other related interventions. A total of 84 patients received TPCs at Inova Fairfax Hospital (Falls Church, Virginia) by the thoracic surgery service. These patients with MPEs received a TPC instead of a surgical intervention because of a Karnofsky score less than 80, trapped lung physiology, or patient preference. At final follow-up, 8 of the 84 patients were still alive with continued TPC drainage. One patient was excluded from all analysis for the TPC being placed for benign disease. As a result, 75 patients were included in the final descriptive analysis. All TPCs were placed by the same interventional pulmonologist, under moderate sedation in the bronchoscopy suite or operating room using only PleurXTM catheters (CareFusion).
Following TPC placement, patients were discharged with either home nursing services or hospice care. Patients were given standard of care educational interventions by the nursing staff that instructed patients to drain every other day. Each drainage event was performed until cessation of drainage or chest pressure was experienced, which served as a surrogate for pleural pressure of less than −20 cm/H2O. Seven to 10 days after TPC placement, patients were seen in the outpatient thoracic surgery clinic for follow-up and suture removal.
To determine approximate time to autopleurodesis, proxy variables and measurements were utilized. A patient was deemed to have achieved autopleurodesis if the TPC was removed prior to death indicated by drainage of less than 50 milliliters of fluid observed on three consecutive drainages. Thoracic ultrasound was performed immediately prior to removal to confirm absence of retained, loculated fluid. A patient was not considered to reach autopleurodesis if they expired with TPC in place or if a patient was living at the follow-up time in September 2016. Only patients with a TPC removal date or death date occurring within the 2-year period were considered in final analysis for average time to autopleurodesis or death. Patients were considered to have a “bilateral TPC placement” if both a left and a right TPC were placed on the same day or within 7 days of the initial TPC placement. To best describe the patient experience and outcomes of the catheter, data points of interest populated were: duration of time to reach autopleurodesis or death, type and number of complications, treatment with chemotherapy while TPC was in place, and need for tissue plasminogen activator (tPA) instillation for loculated fluid resulting in poor drainage or obstructed catheter seen on thoracic ultrasound. Five milligrams of tPA was instilled for 2 hours into the catheters followed by drainage.
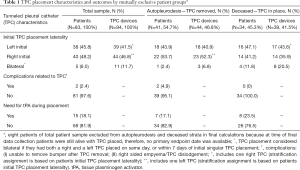
Given the project’s hypothesis generating nature, the calculated patient information is purely descriptive, and no statistical, comparative tests were performed. Proportions, frequencies, means, standard deviations, medians, quartiles and ranges were utilized to characterize the patient sample. A binary variable was created for carcinoma type to group lung-related carcinomas followed by all other carcinomas. One patient with mesothelioma was included in the lung carcinoma stratum. For TPC frequencies by outcome groups in Table 1, patients and TPCs are assigned to mutually exclusive laterality stratums based on which side first TPC is placed on, if a patient had more than one TPC placed. Bilateral placements were also considered separately. A small proportion of patients received a second TPC in the contralateral side after the 7-day definition of bilateral placement.
Full table
Results
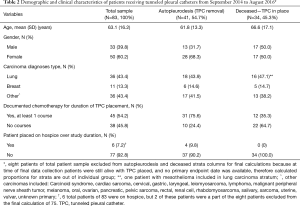
Table 2 characterizes the study sample consisting of 83 patients, where 33 were male and 50 were female. The mean age of the sample was 63.1 years. There were 24 different types of carcinomas represented in the sample, the most common being lung cancer (n=36, 43.4%) followed by breast cancer (n=11, 13.3%). Forty-five patients (54.2%) were treated with at least one course of chemotherapy while the TPC was in place (n=45, 54.2%). By September 2016, 50 patients (60.2%) had deceased while eight patients (9.6%) were still alive with continued TPC drainage. In total, 94 TPCs were placed in 83 patients, including five patients (6.0%) with bilateral TPC placements.
Full table
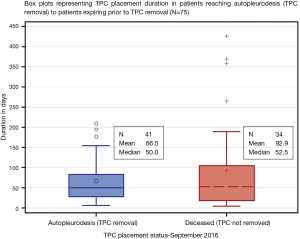
Only 75 patients (90.4%) with 84 total devices were included in final analysis based on available endpoint dates (Figure 1). Where 41 patients (54.7%) reached autopleurodesis, indicated by TPC removal date and 34 patients (45.3%) expired with TPC in place (Table 1). Four out of 5 patients with bilateral placements deceased with TPC in place. A total of 2 (2.4%) complications documented occurred in two individual patients, and were attributed to the presence of the TPC. Both complications were infectious and resulted in empyema formation. No patients died as a direct result of TPC complication and no complications were noted at time of catheter placement. No catheters required replacement for inability to drain.
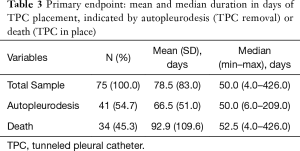
For patients reaching autopleurodesis (TPC removal), it took a median of 7.1 weeks or 50.0 days. It took patients a median of 7.5 weeks or 52.5 days to expire with TPC in, after initial placement (Table 3, Figure 2). Median times were favored over the mean because the distribution of time to autopleurodesis or death was not normally distributed (Figure 3). There are extreme outliers skewing the mean duration of TPC placement away from the true estimate, rendering the mean value less representative.
Full table

Forty-three patients received chemotherapy while their TPC was in place. Of the 41 patients reaching autopleurodesis, 31 received chemotherapy, while only 12 patients in the group failing to reach autopleurodesis (n=34) received chemotherapy.
A total of 15 patients required instillation of tPA for poor drainage of loculated pleural fluid based on thoracic ultrasound or for an obstructed TPC. Five milligrams of tPA, mixed in 40 milliliters of normal saline was instilled through the TPC, left in the pleural space for 2 hours followed by drainage. Seven of these patients were in the autopleurodesis group, while 8 were patients failing to reach autopleurodesis, and expiring prior to TPC removal.
Discussion
MPE are associated with significant morbidity and commonly have a poor prognosis. The goal of TPC placement is to alleviate symptoms such as shortness of breath in patients with MPEs, and to achieve definitive autopleurodesis. Our study adds to the growing body of literature describing the safety and effectiveness of TPCs.
The 54.7% of the current patient sample who achieved autopleurodesis is comparable to previous studies that describe the use of TPCs in MPE, with every other day drainage. Previous TPC studies investigating MPEs typically attain a rate of autopleurodesis rate between 50–70% (8,10,11,12) Additionally, the median time observed to reach autopleurodesis of 50.0 days or 7.1 weeks is comparable to accepted data of 52–56 days (4,8).
No pneumothoraces resulted from TPC placement as indicated by postoperative chest X-ray. Additionally, no excessive bleeding occurred during TPC placement, defined as bleeding requiring blood transfusion or additional surgical intervention. Incidence of infectious complications following TPC placement in the form of empyema was seen in only two patients (2.7%). One case resulted from an accidental dislodgement of the TPC by the patient, whom did not seek medical treatment, which lead to cellulitis with extension of infection into the pleural space. Neither case required surgical intervention and no deaths were attributed to empyema secondary to TPCs. Our reported infection rate is lower than that of published accounts of other high-volume centers (6). Procedural nurses educating patients on proper TPC drainage and care may account for the lower infection rate in our patient sample. This instructive intervention included an extensive one-on-one teaching session by nurses prior to TPC placement, paired with home nursing visits within 7 days following placement. This ensured appropriate drainage and sterile technique by patients and caregivers. Only one patient (2.4%) in the autopleurodesis group had recurrence of pleural fluid on the same side after catheter removal, necessitating repeat placement of TPC nearly 8 months later. Systematic review data reveal a 5.1% incidence of requiring repeat TPC placement after catheter was removed (8).
Instillation of tPA into TPCs may improve drainage in loculated pleural effusions. Fifteen patients (20.0%) of the 75 in our cohort required tPA instillation for poor drainage due to loculated effusion seen on thoracic ultrasound or for a clotted TPC. Seven patients required tPA instillation in the group that attained autopleurodesis, while eight patients required tPA instillation in the group that did not reach autopleurodesis and who were deceased prior to TPC removal. The effect of tPA instillation into TPCs with loculated pleural effusions will require future studies and analysis.
A single chi-square test to review potential differences between the autopleurodesis group and deceased prior to autopleurodesis group, in relation to simultaneous chemotherapy with TPC in place was conducted. Of the 41 patients achieving autopleurodesis, 31 (75.6%) received at least one course of chemotherapy while TPC was in place. Of the 34 patients who did not reach autopleurodesis, and deceased prior to TPC removal, only 12 (35.3%) received chemotherapy while the TPC was in place (P=0.0004). These numbers may reveal some uncontrolled bias such as the fact that patients expiring with TPC in place are potentially more ill, or further along in the disease process, thus being too sick to receive chemotherapy, compared to the autopleurodesis group. Despite this probable influence, the significance of the effect of chemotherapy on achieving autopleurodesis, remains intriguing, and should be tracked and analyzed in future observational studies.
A strong suit of this pilot study is the quick accumulation of patients into a moderate-to-large sample size, which increases generalizability and current clinical relevance. This is a striking comparison to other institutions which have a lower-case volume and slower subject accrual rates, creating wide sample variability. The 2-year study duration allowed investigators adequate accrual and follow-up time to identify exploratory patient outcomes. The design dynamically added patients to the cohort, and provided easy access to vital TPC related data, events, and disease outcomes without complications of a prospective follow-up. Though descriptive in nature, identification of patient and clinical characteristics will provide a strong foundation to guide further data collection and analysis in the field.
Limitations of our study are related to an initial exploratory nature and utilization of retrospective patient chart data of a single-center cohort. Other clinically relevant covariates were not accounted for, such as medical comorbidities. While it is standard of care for patients to be instructed to drain every other day, the drainage regimen was altered on occasion based on symptoms and patient convenience. Currently, there are insufficient data in medical records to reliably identify varying drainage patterns, and any available data is likely self-reported. This can cause confounding bias and should be properly accounted for in future prospective studies. Finally, since TPCs are generally placed for end-of-life palliation in patients, the proportion of patients in the failure to achieve autopleurodesis group, may be higher than expected since TPCs could have been placed in patients within days of death for palliative care.
Conclusions
Our exploratory study reports clinical outcomes and complications of TPCs in a high-volume institution and adds to a growing body of literature describing the use and effectiveness of TPCs. We show a time to autopleurodesis rate that aligns with current literature, and a lower incidence of complications. Only one patient required repeat TPC placement due to recurrence of a pleural effusion after catheter removal. Additionally, our study highlights the impact of tPA instillation into loculated pleural effusions and the significance that chemotherapy could play in patients achieving autopleurodesis. We hope this study will provide a springboard for further investigations regarding the effects and benefits of TPCs in MPEs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Inova Institutional Review Board (IRB approval #16-2364) and written informed consent was waived as it’s a retrospective quality project.
References
- Meyer PC. Metastatic carcinoma of the pleura. Thorax 1966;21:437-43. [Crossref] [PubMed]
- Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695-702. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Thomas R, Jenkins S, Eastwood PR, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015;21:338-45. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Skalski JH, et al. The Use of Indwelling Tunneled Pleural Catheters for Recurrent Pleural Effusions in Patients With Hematologic Malignancies: A Multicenter Study. Chest 2015;148:752-8. [Crossref] [PubMed]
- Pollak JS. Malignant pleural effusions: treatment with tunneled long-term drainage catheters. Curr Opin Pulm Med 2002;8:302-7. [Crossref] [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Al-Halfawy A, Light R. Safety and efficacy of using a surgivac pump for the drainage of chronic indwelling pleural catheters in malignant pleural effusions. Respirology 2008;13:461-4. [Crossref] [PubMed]
Cite this article as: Mahajan AK, Collins DT, Powers C, Khandhar SJ. Tunneled pleural catheters for management of malignant pleural effusions: a 2-year review of outcomes at a high-volume center. Shanghai Chest 2018;2:27.