Technical aspects of extrapleural pneumonectomy
Introduction
The technique of extrapleural pneumonectomy (EPP) for the treatment of tuberculous empyema was first described by Sarot in 1949 (1). Butchart and coll. published the first series on EPP for the treatment of malignant pleural mesothelioma (MPM) in 1976 (2) and, since then, it has been performed almost exclusively for MPM, with a decreasing mortality from 31% to 3.4% (3).
MPM is an extremely aggressive disease for which single modality therapy alone is largely ineffective (4) thus requiring multimodality approach to optimize oncological results (5-7).
The main target of surgery is to obtain a macroscopic radical resection that—in case of EPP—means en-bloc resection of lung, pleura, pericardium, and diaphragm with radical lymphadenectomy, followed by reconstruction of diaphragm and pericardium (8).
Although progress and improvements made during the last 40 years, EPP still remains an extremely challenging procedure—both from the technical and the clinical point of view—and it should therefore only be performed at high volume centers by experienced surgeons and anesthesiologists.
Oncologic principles
Only patients suffering from early stage resectable MPM should be considered for EPP; patients with mediastinal or extrathoracic nodal involvement are not suitable for EPP.
Tumor histology is a key point influencing survival together with tumor stage: epithelioid MPM confers the best prognosis while sarcomatous and mixed histologies are more aggressive, thus being EPP indicated in early stage epithelioid MPM (9,10).
Preoperative radiological evaluation is crucial to disclose a potentially resectable disease, being computed tomography (CT) of the chest and upper abdomen and magnetic resonance imaging (MRI) the most widely used modalities.
Positron emission tomography (PET) is useful to determine if a patient presents nodal involvement, contralateral disease or—rarely—distant metastasis (10).
EBUS TBNA or mediastinoscopy are used to prove nodal involvement; occasionally laparoscopy may identify transdiaphragmatic involvement that would preclude resection.
Preoperative assessment
Candidates to EPP should have a normal performance status (PS) ranging between 0 and 1; patients with a predicted postoperative FEV 1 (ppoFEV1) less than 1 liter or than 30% of theoretical value present a higher risk of perioperative complications and should undergo cardiopulmonary exercise testing (CPET) to further explore their cardiorespiratory function.
Arterial blood gases should disclose at least room air PaO2 >65 mmHg and PaCO2 <45 mmHg. Ejection fraction less than 40% as well as mean pulmonary artery pressure >30 mmHg may significantly increase perioperative risks (3).
Right EPP
After induction of general anesthesia, a left-sided double lumen endotracheal tube is positioned fiber optically; a nasogastric tube is also required to allow palpation of the esophagus during the posterior extrapleural dissection and lymphadenectomy.
The patient is placed in the left lateral decubitus position.
A first standard right lateral total muscle sparing thoracotomy is performed in the 5th or 4th intercostal space incorporating—if possible—all previous biopsy sites; otherwise they have to be removed during the second lower thoracotomy or separately.
After entering the chest cavity, an extrapleural plane is found superior and inferior to the thoracotomy; the extrapleural dissection starts from the upper part of the chest cavity, avoiding to injure the internal mammary vessels as well as the subclavian vessels which can determine significant bleeding.
Sharp and blunt dissection continues apically with great care to avoid injuries to the superior vena cava and to the azygos vein. Retro-phrenic longitudinal pericardiotomy is performed to exclude cardiac involvement: the right main pulmonary artery is then intrapericardially dissected and isolated on vessel slings, as well as both pulmonary veins. Pericardiotomy is then completed at the pericardiopleural attachment overlying the junction of the inferior vena cava and hepatic veins.
A second standard right lateral total muscle sparing thoracotomy is performed to resect the diaphragm, usually in the 8th or 9th intercostal space, depending on chest cavity depth and possible diaphragm rising.
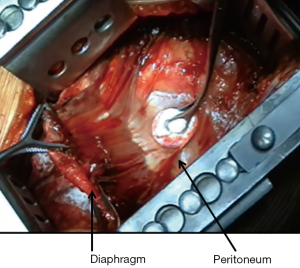
The diaphragm is excised starting anteriorly and circumferentially, without entering the peritoneum if feasible (Figure 1); the diaphragm is divided over the inferior vena cava with great care not to damage hepatic veins; the dissection is extended posteriorly leaving a rim of health tissue—if not involved by tumor—to secure the reconstruction patch; in case of complete diaphragm involvement, the patch will then be sutured directly to the ribs and to the vertebral transverse processes.
Sub carinal and right paratracheal lymphadenectomy is standardly performed.
Superior pulmonary vein, right pulmonary artery and inferior pulmonary vein are then ligated and divided with 45 mm vascular staplers (Endo GIATM 45 Covidien-Medtronic Minneapolis, MN, USA) intrapericardially; after the vessels division, the pericardium is opened posteriorly; after hilar lymphadenectomy, the right mainstem bronchus is stapled with a 30-mm TA stapler (Premium Multifire TATM 30-V3 Covidien-Medtronic Minneapolis, MN, USA) and cut with knife; the specimen is finally removed.
If possible a tissue flap (muscle, thymus, azygos vein, fat tissue if still available) is developed to protect the bronchial stump and prevent broncho-pleural fistula.
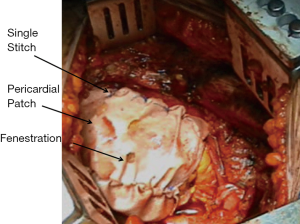
The pericardium is usually reconstructed with a bovine pericardium patch utilizing only interrupted 2-0 polyprolene stitches and avoiding running suture to prevent cardiac herniation in case of suture disruption; the patch should be fenestrated and not too narrow in order to prevent tamponade in case of cardiac bleeding and constrictive physiology (Figure 2).
Diaphragm can be reconstructed with 2-mm soft tissue polytetrafluorethylene patch (Gore-Tex, Inc., Flagstaff, AZ, USA) or bovine pericardium using interrupted 1 polyprolene sutures.
Medially, the diaphragmatic patch is sutured to the pericardial patch to avoid impaired venous return of the inferior vena cava; the diaphragmatic patch prevents intrathoracic herniation of abdominal organs although—on the right side—this complications is quite infrequent due to the presence of the liver.
A very meticulous and long-lasting haemostasis—performed by several techniques and devices—(washings, laser, thrombin solutions, sealants, electrocautery) is needed because postoperative haemothorax is one of the most common complications following EPP.
A 32 or 28 French chest tube is placed—depending on chest cavity size—and secured with silk purse string. The chest cavity is then standardly closed to ensure airtight.
Right EPP via median sternotomy has been also described hypothesizing less postoperative pain and morbidity; in this setting the pericardium is reconstructed early in the operation, occasionally before excision of the diaphragm, to minimize cardiac manipulation and haemodynamic compromise (11).
It has been widely discussed whether routinely replacing the diaphragm introduces an unnecessary cause of postoperative complications; however—given the difficulty in determining diaphragmatic involvement intraoperatively, and the detrimental effect on survival of macrospic residual disease—resection of the diaphragm is strongly recommended as part of EPP (12).
Left EPP
After induction of general anesthesia, a right-sided double lumen endotracheal tube is positioned fiber optically to avoid dysventilation of the right upper lobe; a nasogastric tube is also required to allow palpation of the esophagus during the posterior extrapleural dissection and lymphadenectomy.
The patient is placed in the right lateral decubitus position.
Almost all the surgical steps are the same described for right EPP, with some significant differences.
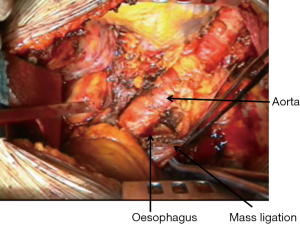
During the dissection of the posterior aspect of the specimen great care must be taken to find the correct plane in the periaortic region to avoid avulsion of intercostal vessels or injury to the aorta (Figure 3); however, the rigidity of the aorta usually offers a better cleavage plane than superior vena cava and azygos vein.
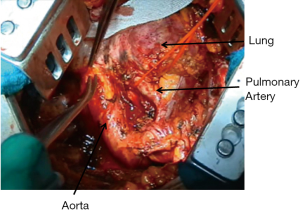
The oesophagus should be clearly identified before proceeding with sharp or blunt dissection in order to avoid damages during extrapleural dissection or subcarinal lymphadenectomy.
Although left-sided bronchopleural fistula is quite uncommon, bronchial stump coverage is anyway recommended whenever possible.
Reconstruction of the esophageal hiatus should be carefully performed to avoid—on one hand—intrathoracic herniation of the stomach and—on the other hand—postoperative dysphagia due to a too tight reconstruction.
Thoracic duct mass ligation is recommended to prevent postoperative chylothorax (Figure 4).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David Waller and Annabel Sharkey) for the series “Mesothelioma Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.03.14). The series “Mesothelioma Surgery” was commissioned by the editorial office without any funding or sponsorship. LS serves as an unpaid editorial board member of Shanghai Chest from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarot IA. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949;4:173-223. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
-
Extrapleural Pneumonectomy - DaSilva MC, Sugarbaker DJ. Technique of extrapleural pneumonectomy. Operative Techniques in Thoracic and Cardiovascular surgery. Available online: http://www.optechtcs.com/article/S1522-2942(10)00135-2/pdf
- Facchetti G, Petrella F, Spaggiari L, et al. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur J Med Chem 2017;142:266-70. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Brambilla D, et al. Induction chemotherapy, extrapleural pneumonectomy and adjuvant radiotherapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2017;52:975-81. [Crossref] [PubMed]
- Petrella F, Coccè V, Masia C, et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed Pharmacother 2017;87:755-8. [Crossref] [PubMed]
- Sugarbaker DJ. Macroscopic complete resection: the goal of primary surgery in multimodality therapy for pleural mesothelioma. J Thorac Oncol 2006;1:175-6. [Crossref] [PubMed]
- Sugarbaker DJ, Mentzer SJ, Strauss G. Extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Thorac Surg 1992;54:941-6. [Crossref] [PubMed]
- Gerbaudo VH, Sugarbaker DJ, Britz-Cunningham S, et al. Assessment of malignant pleural mesothelioma with (18)F-FDG dual-head gamma-camera coincidence imaging: comparison with histopathology. J Nucl Med 2002;43:1144-9. [PubMed]
- Edwards JG, Martin-Ucar AE, Stewart DJ, et al. Right extrapleural pneumonectomy for malignant mesothelioma via median sternotomy or thoracotomy? Short- and long-term results. Eur J Cardiothorac Surg 2007;31:759-64. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
Cite this article as: Petrella F, Casiraghi M, Spaggiari L. Technical aspects of extrapleural pneumonectomy. Shanghai Chest 2018;2:22.