Gender-based differences of abdominal adipose tissue distribution in non-small cell lung cancer patients
Introduction
Lung cancer is a malignant tumor with high lethality, responsible for about 28% of all cancer deaths (1). Its etiology integrates a variety of environmental stimuli and factors; in particular, the association between smoking and lung cancer is clear (2-5). Today, scientific interest is geared towards other potential risk factors, including occupational, lifestyle, dietary and even reproductive covariates (6-8). Specifically, concerning the role of obesity, white adipose tissue (WAT) acts as an endocrine organ, active contributor to body homeostasis rather than just being a fat deposit, including a role in immunity and inflammation (9,10). It has been reported that the prevalence and the mortality of several types of cancer, such us breast, endometrium, colorectal and prostate cancer, increase with obesity (11-13). The precise mechanism that explains how obesity promotes these diseases is still unclear; however, recent studies suggest that the accumulation of WAT in the abdominal cavity [i.e., visceral adipose tissue (VAT)] may play a key role in this relationship (14). VAT display differences in anatomic, cellular and molecular compositions, showing higher hormonal and metabolic activities than subcutaneous fat tissue (SAT) (15,16). VAT distribution in men and women is determined by both sex steroids, androgens and estrogens, with a predominance of androgen action, that increase during menopause and in women with central obesity (17-20). In particular, visceral adipocyte-secreted growth factors, proinflammatory cytokines, and adipokines, are mediating factors associated with the carcinogenesis of obesity-related tumours (21). Studies have showed how VAT, with the emerging carcinogenic properties of adipokines, increase colorectal cancer risk (14,22-24).
Regarding lung cancer, it is not considered to be mainly an obesity related cancer and some authors also reported that obesity decreased lung cancer mortality independently of smoking status and might have a potential favorable effect on lung cancer survival (25-28). However, some recent studies have underlined an adipokines’ functional role in lung cancer development and progression, especially for non-small cell lung cancer (NSCLC) (29). According to the literature, the adipokines levels might provide diagnostic and prognostic information for lung cancer, making them potential mediators of the complex and still unclear multistep lung carcinogenesis (29,30).
To the best of our knowledge, the distribution of VAT and SAT has never been explored in detail in a selective group of patients with NSCLC. In this study, using a quantitative CT imaging-based approach, we aimed to investigate the differences of abdominal adipose tissue distribution in patients with NSCLC according to gender.
Methods
Patients
In this retrospective study we included two groups, the patients group and the control group. All of the subjects enrolled underwent a CT examination in our institution between April 2006 and April 2016. In the patients group, a total of 66 patients with NSCLC at first diagnosis were included [male: 45, mean age: 67.3 years (range, 51.0–88.0 years); female: 21, mean age: 62.4 years (range, 44.0–84.0 years)]. All the patients received a biopsy and a histologically confirmed diagnosis of NSCLC (male: 33 adenocarcinomas, 8 squamous cell carcinomas and 4 NSCLCs; female: 19 adenocarcinomas, 1 squamous cell carcinomas and 1 NSCLCs). For the patients with NSCLC all CT images were acquired for disease staging at the first diagnosis, prior to any medical or surgical treatment for the oncologic disease.
Owing to the fact that the abdominal CT is usually not performed in healthy subjects, as a control group we included 70 patients who have undergone a chest-abdomen CT for pre-operative cardiovascular surgery planning [male: 42, mean age: 62.7 years (range, 40.0–83.0 years); female: 28, mean age: 66.4 years (range, 39.0–83.0 years)]. The specific type of cardiac surgery that the patients received was as follows: 28 mitral valve replacement, 22 aortic valve replacement, 2 mitral and tricuspid valve replacement, 1 mitral and aortic valve replacement, 2 left atrial myxoma resection, 1 right atrial myxoma resection, 4 combined coronary artery bypass and mitral valve replacement, 2 combined coronary artery bypass and aortic valve replacement, 1 combined coronary artery bypass with mitral and tricuspid valve replacement, 5 aortic valve and ascending aorta replacement, 1 carotid endarterectomy and 1 ostium secundum atrial septal defect repair. None of the patients included in the control group had a history of malignancies.
The study was approved by the local ethical committee and was conducted in accordance with the Declaration of Helsinki.
CT analysis
CT images were acquired using the clinical scanner Somatom, Sensation 64, Siemens, Forcheim, Germany. In order to calculate TAA, VAA and SAA, OsiriX MD v.2.6 was used to analyze cross-sectional CT images. All measurements were obtained as area (cm2), on the axial plane at the level of L3 (Figure 1), following a similar approach used elsewhere (31). First, region of interest (ROIs) of TAA were segmented and calculated for each subject using a function of OsiriX MD v.2.6. Then, ROIs of SAA were generated and calculated by removing ROIs of VAA from those of TAA. Finally, values of VAA (cm2) were calculated for each patient by subtracting values of SAA from those of TAA.
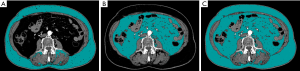
All segmentations were visually inspected by two experienced radiologists by consensus, to improve the accuracy of the measurements.
Statistical analysis
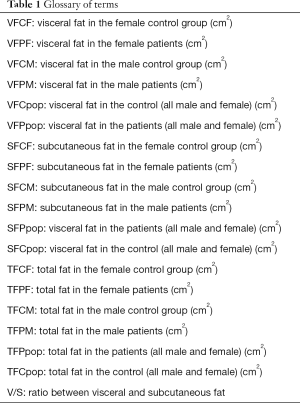
Simple regression analysis was carried out to estimate the correlation between age and the following parameters: VFCF, VFPF, VFCM, VFPM, SFCF, SFPF, SFCM, SFPM, TFCF, TFPF, TFCM, and TFPM (see glossary of terms, Table 1). The Pearson’s correlation coefficient (r) and the significance of the simple regressions (ρ) were calculated.
Furthermore, the values of the above-mentioned parameters were compared between patients and control group. The differences were analyzed using the Student’s t-test (P<0.05).
Full table
All the statistics were developed in MATLAB® (MathWorks, Inc., Natick, Massachusetts, USA) environment.
Results
Comparison of the parameters of interest between control and patients
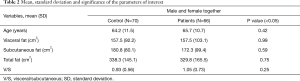
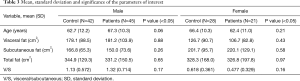
Descriptive statistics which included mean and standard deviation (SD) were obtained for each group by comparing controls and patients, both considering male and female together (Table 2) and separately (Table 3).
Full table
Full table
The differences between patients and control groups of visceral, subcutaneous and total fat were not significant (Tables 2,3). Considering male and female subjects together, the amount of visceral fat was similar between patients and controls; moreover, the controls showed higher values of subcutaneous and consequently total abdominal fat.
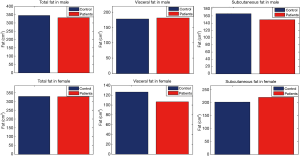
With male and female subjects separately, data show that the mean value of the visceral fat was lower in patients than in control group in female, on the other hand the subcutaneous was higher in patients than controls. Regarding male group, the subcutaneous fat was lower in patients than in controls and visceral fat was slightly higher in patients than controls (Figure 2).
The ratio between visceral and subcutaneous fat (V/S) was also calculated for all groups and reported in Tables 2,3.
Considering male and female together we found a slightly higher V/S ratio in patients than control group (Table 2).
Considering male and female separately, in the female group, the V/S ratio was higher in the control group than in patients. Conversely, in the male group we found a higher V/S ratio in patients with respect to the controls (Table 3).
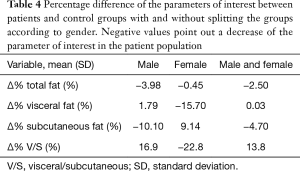
We also calculated the percentage changes (Δ%) of visceral, subcutaneous and total fat, as well as the change of the ratio between visceral and subcutaneous fat, as follows:
where XP is the mean of the parameter of interest calculated on the patient’s population, and XC is the mean of the parameter of interest calculated on the control. These values are reported in Table 4.
Full table
Simple regression
In Table 5, the Pearson’s coefficient and the significance of the simple regression (ρ) between age and the parameters of interests (i.e., VFCF, VFPF, VFCM, VFPM, VFCpop, VFPpop, SFCF, SFPF, SFCM, SFPM, SFCpop, SFPpop, TFCF, TFPF, TFCM, TFPM, TFCpop, and TFPpop) are reported.
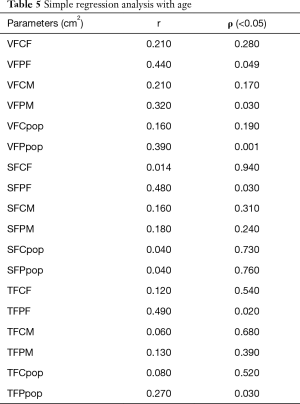
Full table
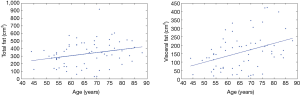
In Figure 3, the plots of the two statistically significant regressions are reported considering male and female together. These two parameters (i.e., VFPpop and TFPpop) increased with age.
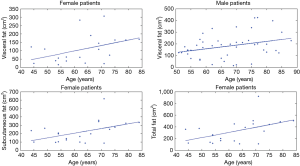
In Figure 4, the plots of the four statistically significant regressions are reported dividing the population between male and female. These four parameters (i.e., VFPF, VFPM, SFPF, and TFPF) increased with age.
Discussion
In this study, we have assessed the distribution of abdominal adipose tissue in patients with NSCLC. With male and female subjects together, we found a similar amount of VAT in patients and controls, conversely, SAT and total abdominal adipose tissue were increased in control group. These differences, although not statistically significant, suggest a preservation of VAT in patients with respect to the amount of subcutaneous and total abdominal fat tissue. Indeed, we found a slightly higher V/S ratio in patients than control.
According to the literature, an association between low body mass index (BMI) and lung cancer risk has been suggested (32). The measure of BMI, used in epidemiologic studies as a proxy measure of obesity, was found to be positively associated with increased risk of cancer in several sites including colon, breast in postmenopausal women, endometrium, kidney (renal cell), stomach, pancreas, gallbladder, liver, and possibly other cancers (33). Conversely, for lung cancer an inverse association with BMI was reported in several studies, suggesting that obesity might be a preventive factor for the development of lung cancer (34-42). However, data are controversial on this subject since some studies have failed to find such an association, in other the association disappeared when habits and health status were taken into account and a few studies reported a positive association between BMI and lung cancer incidence (25,26,43-46).
Our results suggest that the inverse association between lung cancer risk and BMI observed in several studies, as for the abdominal fat contribution to BMI, might be mainly due to a reduction of the amount of subcutaneous with a preservation of visceral fat in patients.
Moreover, with separation of the groups between male and female, we found a different pattern of abdominal fat distributions according to gender. In the male group we found that the visceral fat was higher and the subcutaneous lower in patients than in controls group, conversely, in the female group the visceral fat was lower and the subcutaneous higher in patients than in controls group.
Many studies have reported gender differences in clinical presentation and the biology of lung cancer (47), elucidating major differences between men and women in development, physiology, predilection to and outcomes in lung diseases (48). Body fat mass in men and women is determined by both androgens and estrogens, which play important roles resulting in different patterns of total abdominal adipose tissue distribution (20). This gender-based distribution of fat is based on the assumption that women have a greater amount of peripherally located subcutaneous fat and, conversely, men show a greater amount of centrally located visceral fat (49).
In premenopausal women estrogens are produced through the conversion from androgens, which is catalyzed by aromatase enzymes, that are highly expressed in placenta and in granulose cells of ovarian follicles (50) and, at lower levels, in several nonglandular tissues including subcutaneous fat, liver, muscle, brain, normal breast and breast cancer tissue (51). In postmenopausal women, estrogen production is solely from nonglandular sources, in particular from subcutaneous fat with a peripheral conversion from androgens (52).
VAT has a predominance of androgen action over that of estrogen (17,18). Moreover, women’s VAT mass increase when their estrogen concentrations decreased, and androgen concentrations increased or in case of an excess of androgens and androgens administration (53-55). In men VAT increase when androgen concentrations decrease (56).
Therefore, in our study, this different gender-based pattern of abdominal fat distribution might be related to a different hormone-linked status between men and female patients with NSCLC. Indeed, it is known that estrogen play an important role in initiating and promoting lung cancer (57).
Lastly, exploring the correlations, we found that visceral fat increased with age in patients considering all the patients together as well as dividing the population between male and female. Total abdominal fat increased with age in all patients together and, only for female patients, subcutaneous and total abdominal fat increased with age.
It is known that body fat percentage increases during life, with peaks during the fifth and seventh decade of life and subsequently remain constant or decrease slightly (58,59). Aging may act on the adipose tissue function and determine a change of adipokines production from adipocytes by regulating pre-adipocytes and adipose tissue infiltration by macrophages (60). However, several factors including nutrition, physical activity, menopausal status and diseases, might influence the abdominal fat distribution according to age.
The relationship between age and abdominal fat distribution has been poorly explored in NSCLC patients. A recent study on elderly patients reported that total adipose tissue and VAT were positively associated with cancer risk among women whereas no such an association was found among men (61). Total adipose tissue was positively associated with obesity-related cancer risk among women and VAT was positively associated with obesity-related cancer risk among men (61). The results of our study are in line with these findings and reinforce the concept of a differential gender-based relationships among abdominal fat distribution, age and cancer, specifically NSCLC. However, the biological basis of this complex relationship is not fully understood and deserve further investigations. Additional studies are needed to understand whether the abdominal fat distribution at first diagnosis might influence to the disease staging, response to treatment and prognosis in NSCLC. Moreover, it is unknown if the observed pattern of abdominal fat might be present in other histotypes of lung cancer such as small cell lung cancer.
Given the retrospective design of our study, as limitation of it should be considered that we could not obtain some clinical information of the enrolled patients such as BMI, hormonal status, pre- or post-menopausal status and smoking rates. Thus, it was not possible to include this factors in our analysis as covariates. Future studies taking in to account these factors will be beneficial in order to understand the impact of it, in any, on our results.
Conclusions
The results of this study are in line with the concept of differential gender-based relationships among abdominal fat distribution, age and NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethical committee of Università Campus Bio-Medico di Roma and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality--beyond established causes. N Engl J Med 2015;372:631-40. [Crossref] [PubMed]
- Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351-64. [Crossref] [PubMed]
- Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med 1999;340:1773-80. [Crossref] [PubMed]
- Fielding JE. Smoking: health effects and control (1). N Engl J Med 1985;313:491-8. [Crossref] [PubMed]
- Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol 2007;25:5785-92. [Crossref] [PubMed]
- Kubik A, Zatloukal P, Tomasek L, et al. Lung cancer risk among nonsmoking women in relation to diet and physical activity. Neoplasma 2004;51:136-43. [PubMed]
- Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, et al. Consumption of tea and coffee and the risk of lung cancer in cigarette-smoking men: a case-control study in Uruguay. Lung Cancer 1998;19:101-7. [Crossref] [PubMed]
- Lago F, Dieguez C, Gomez-Reino J, et al. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 2007;3:716-24. [Crossref] [PubMed]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347-55. [Crossref] [PubMed]
- Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr 2002;132:3451S-3455S. [Crossref] [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [Crossref] [PubMed]
- Marmot M, Atinmo T, Byers T, et al. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR, 2007.
- Lee JY, Lee HS, Lee DC, et al. Visceral fat accumulation is associated with colorectal cancer in postmenopausal women. PLoS One 2014;9:e110587. [Crossref] [PubMed]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11-8. [Crossref] [PubMed]
- Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39-48. [Crossref] [PubMed]
- Wajchenberg BL, Giannella-Neto D, da Silva ME, et al. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 2002;34:616-21. [Crossref] [PubMed]
- Korhonen S, Hippelainen M, Vanhala M, et al. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril 2003;79:1327-34. [Crossref] [PubMed]
- Kirschner MA, Samojlik E, Drejka M, et al. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab 1990;70:473-9. [Crossref] [PubMed]
- Battisti S, Guida FM, Coppa F, et al. Modification of abdominal fat distribution after aromatase inhibitor therapy in breast cancer patients visualized using 3-D computed tomography volumetry. Clin Breast Cancer 2014;14:365-70. [Crossref] [PubMed]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881-7. [Crossref] [PubMed]
- Oh TH, Byeon JS, Myung SJ, et al. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol 2008;23:411-7. [Crossref] [PubMed]
- Yamamoto S, Nakagawa T, Matsushita Y, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care 2010;33:184-9. [Crossref] [PubMed]
- Erarslan E, Turkay C, Koktener A, et al. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci 2009;54:862-8. [Crossref] [PubMed]
- Drinkard CR, Sellers TA, Potter JD, et al. Association of body mass index and body fat distribution with risk of lung cancer in older women. Am J Epidemiol 1995;142:600-7. [Crossref] [PubMed]
- Kanashiki M, Sairenchi T, Saito Y, et al. Body mass index and lung cancer: a case-control study of subjects participating in a mass-screening program. Chest 2005;128:1490-6. [Crossref] [PubMed]
- Leung CC, Lam TH, Yew WW, et al. Lower lung cancer mortality in obesity. Int J Epidemiol 2011;40:174-82. [Crossref] [PubMed]
- Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res 2011;170:e75-83. [Crossref] [PubMed]
- Ntikoudi E, Kiagia M, Boura P, et al. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev 2014;40:22-30. [Crossref] [PubMed]
- Kerenidi T, Lada M, Tsaroucha A, et al. Clinical significance of serum adipokines levels in lung cancer. Med Oncol 2013;30:507. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer 2013;132:1162-9. [Crossref] [PubMed]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91. [Crossref] [PubMed]
- Knekt P, Heliovaara M, Rissanen A, et al. Leanness and lung-cancer risk. Int J Cancer 1991;49:208-13. [Crossref] [PubMed]
- Kabat GC, Wynder EL. Body mass index and lung cancer risk. Am J Epidemiol 1992;135:769-74. [Crossref] [PubMed]
- Goodman MT, Wilkens LR. Relation of body size and the risk of lung cancer. Nutr Cancer 1993;20:179-86. [Crossref] [PubMed]
- Chyou PH, Nomura AM, Stemmermann GN. A prospective study of weight, body mass index and other anthropometric measurements in relation to site-specific cancers. Int J Cancer 1994;57:313-7. [Crossref] [PubMed]
- Olson JE, Yang P, Schmitz K, et al. Differential association of body mass index and fat distribution with three major histologic types of lung cancer: evidence from a cohort of older women. Am J Epidemiol 2002;156:606-15. [Crossref] [PubMed]
- Kubik AK, Zatloukal P, Tomasek L, et al. Dietary habits and lung cancer risk among non-smoking women. Eur J Cancer Prev 2004;13:471-80. [Crossref] [PubMed]
- Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006;17:901-9. [Crossref] [PubMed]
- Liu E, Wang X, Yuan J, et al. Association of body mass index with risk of lung cancer: Evidence from a middle-aged male cohort in Shanghai, China. Chin J Clin Oncol 2004;1:90-5. [Crossref]
- Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. [Crossref] [PubMed]
- Rauscher GH, Mayne ST, Janerich DT. Relation between body mass index and lung cancer risk in men and women never and former smokers. Am J Epidemiol 2000;152:506-13. [Crossref] [PubMed]
- Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology 2007;18:607-12. [Crossref] [PubMed]
- Lee IM, Paffenbarger RS Jr. Re: "Body mass index and lung cancer risk Am J Epidemiol 1992;136:1417-9. [Crossref] [PubMed]
- Henley SJ, Flanders WD, Manatunga A, et al. Leanness and lung cancer risk: fact or artifact? Epidemiology 2002;13:268-76. [Crossref] [PubMed]
- Rouquette I, Lauwers-Cances V, Allera C, et al. Characteristics of lung cancer in women: importance of hormonal and growth factors. Lung Cancer 2012;76:280-5. [Crossref] [PubMed]
- Kocurek EG, Hemnes AR. Women's Health and Lung Development and Disease. Obstet Gynecol Clin North Am 2016;43:307-23. [Crossref] [PubMed]
- Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res 2009;2:321-7. [Crossref] [PubMed]
- Labrie F, Simard J, Luu-The V, et al. Structure, regulation and role of 3 beta-hydroxysteroid dehydrogenase, 17 beta-hydroxysteroid dehydrogenase and aromatase enzymes in the formation of sex steroids in classical and peripheral intracrine tissues. Baillieres Clin Endocrinol Metab 1994;8:451-74. [Crossref] [PubMed]
- Labrie F, Luu-The V, Labrie C, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 2003;24:152-82. [Crossref] [PubMed]
- Desta Z, Nguyen A, Flockhart D, et al. Antiestrogen pathway (aromatase inhibitor). Pharmacogenet Genomics 2009;19:554-5. [Crossref] [PubMed]
- Evans DJ, Barth JH, Burke CW. Body fat topography in women with androgen excess. Int J Obes 1988;12:157-62. [PubMed]
- Haarbo J, Marslew U, Gotfredsen A, et al. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism 1991;40:1323-6. [Crossref] [PubMed]
- Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab Rev 1988;4:615-22. [Crossref] [PubMed]
- Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest 1999;22:110-6. [PubMed]
- Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 2006;102:89-96. [Crossref] [PubMed]
- Kuk JL, Saunders TJ, Davidson LE, et al. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009;8:339-48. [Crossref] [PubMed]
- Kyle UG, Genton L, Slosman DO, et al. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition 2001;17:534-41. [Crossref] [PubMed]
- Zamboni M, Rossi AP, Fantin F, et al. Adipose tissue, diet and aging. Mech Ageing Dev 2014;136-137:129-37. [Crossref] [PubMed]
- Murphy RA, Bureyko TF, Miljkovic I, et al. Association of total adiposity and computed tomographic measures of regional adiposity with incident cancer risk: a prospective population-based study of older adults. Appl Physiol Nutr Metab 2014;39:687-92. [Crossref] [PubMed]
Cite this article as: Mallio CA, Greco F, Pacella G, Schena E, Beomonte Zobel B. Gender-based differences of abdominal adipose tissue distribution in non-small cell lung cancer patients. Shanghai Chest 2018;2:20.