
Diaphragmatic plication for eventration or paralysis
Introduction
Etiology
Diaphragmatic eventration are congenital developmental defects affecting the muscular portion of the diaphragm, while the normal attachment to sternum ribs and dorsolumbar spine are maintained. They are rare (incidence <0.05%) and more common among males, affecting more often the left hemidiaphragm. This abnormality occurs as a result of an abnormal migration of myoblasts from the upper cervical somites into two of the four embryological structures that contribute to diaphragm development. Clinically diaphragm eventration is impossible to differentiate from acquired paralysis. In contrast to true diaphragmatic eventration, diaphragm paralysis is a more common acquired condition that can result from a number of abnormalities that affect the neuromuscular axis between the cervical spinal cord and the diaphragm (Table 1)
Table 1
| Level | Causes |
|---|---|
| Central nervous system lesions | Cord transection above C3-4-5, including trauma and transverse myelitis |
| “Hangman’s Fracture”: spinal fracture of both pedicles of the axis vertebra (C2) | |
| Multiple sclerosis | |
| Motor neuron lesions | Amyotrophic lateral sclerosis |
| Poliomyelitis | |
| Spinal muscular atrophy | |
| Nerve root lesions | Cervical spondylosis |
| Trauma—road traffic accident | |
| Peripheral nerve lesions (phrenic) | Traumatic |
| Insertion of central venous line | |
| Pericardial iced cold saline in cardiac surgery | |
| Internal mammary artery harvesting | |
| Thymic or thyroid surgery | |
| Diathermy injury or energy devices | |
| Deliberate phrenic nerve crush to reduce pleural space | |
| Systematic nodal dissection. | |
| Infection—viral neuritis | |
| Benign lymph node invasion (sarcoidosis) | |
| Malignant lymph node invasion | |
| Malignant tumors: lung, thymic, thyroid carcinoma | |
| Pleural plaques | |
| Polyneuropathy | Neuralgic amyotrophy |
| Guillain Barre’ like syndromes | |
| Chronic inflammatory polyneuropathy | |
| Charcot Marie tooth disease | |
| Acute porphyria | |
| Other | Causes |
| Collagen disease or vasculitis | Systemic lupus erythematosus |
| Granulomatosis with polyangitis | |
| Radiotherapy | – |
| Chemotherapy | – |
| Diabetic Neuropathy | – |
| Idiopathic | – |
| Myopathic Lesions | Systemic lupus erythematosus |
| Dermatomyositis | |
| Endocrine | |
| Intensive care myopathy | |
| Amyloidosis | |
| Idiopathic |
Pathology—pathophysiology
Diaphragmatic eventration can be unilateral or bilateral, affecting the whole or only a portion of the hemidiaphragm. The eventrated portion of the diaphragm has an attenuated appearance with microscopically diffuse fibroelastic changes and paucity of muscle fibers. On the contrary a paralysed diaphragm has a normal (although atrophic), amount of muscle fibers. The main symptom for both categories of patients is dyspnoea. In patients with diaphragmatic eventration or paralysis the caudal movement of the diaphragm is less effective (eventration) or absent (paralysis): as a result ventilation is impaired. Among the factors that contribute to the patient’s dyspnoea there is a mismatch in the ventilation/perfusion and loss of pulmonary and chest wall compliance due to impaired perfusion to the basal portion of the lung ipsilateral to the affected hemidiaphragm. This may possibly lead to regional vasoconstriction induced by alveolar hypoxia. Orthopnea develops in some patients with diaphragmatic paralysis as their hemidiaphragm (unlike that of normal persons) is unable to oppose cranial displacement of visceral organs leading to further reduction of lung volumes (1).
Diagnosis and preoperative evaluation
In most cases, patients with eventration or paralysis are asymptomatic and diagnosis is made as an incidental finding on a chest radiograph or as a diagnosis of exclusion in patient with dyspnoea. It is important to determine the start of the symptoms: generally patients with paralysis can recall the moment of the onset of symptoms or when the dyspnoea started or worsened while patients with eventration may not be able to determine a specific starting point.
Pulmonary function test (PFT)
PFT are considered to be an unreliable tool to assess diaphragm function and may not correlate with dyspnea in this setting. However PFTs are essential as they provide objective evaluation. The primary utility of PFT is to monitor changes over time (for example post surgery) (2). Diaphragmatic dysfunction reduced chest-wall compliance and a restrictive patterns is almost always seen. The restriction worsens when supine evidenced by a drop in vital capacity of 30% and 50%. The test is sensitive and has a high negative predictive value: if there is no reduction in FVC when supine, there is probably no significant diaphragmatic paralysis.
Imaging studies
Preoperative evaluation should include a postero-anterior and lateral chest radiograph. In normal condition the right hemidiaphragm is 1 to 2 cm higher than the left. In patients with diaphragmatic eventration or paralysis we expect to see an elevated diaphragm keeping in mind that a variety of pulmonary (i.e., atelectasis and fibrosis) and subdiaphragmatic processes (hepatomegaly, splenomegaly or gastric dilatation) can also cause diaphragmatic elevation.
A computerized tomography of the neck chest and upper abdomen is also a useful tool to distinguish hemidiaphragm elevation secondary to paralysis or eventration with any other cause of diaphragmatic palsy (intrathoracic tumors) or subphrenic process or traumatic hernia or phrenic nerve compression by cervical spinal disease.
Surgical treatment
Indications and contraindications
The only goal of diaphragmatic plication is to manage dyspnea. Therefore surgery is warranted exclusively for symptomatic patients. Indications for plication are based on the presence and severity of respiratory symptoms and their impact on quality of life in symptomatic patients where dyspnea cannot be attributed to another disease with a diaphragmatic elevation that is not caused by another pathologic process other than eventration of paralysis (3). Plication can allow for the resolution of symptoms by achieving the following specific mechanical goals.
- Increased total lung capacity by a more caudal displacement of the diaphragm;
- Decreased redundancy of diaphragmatic surface area with reduction of the paradoxical motion;
- Decreased diaphragmatic compliance allowing intercostal and other accessory respiratory muscles to more effectively create negative and positive intrapleural pressures;
- Increased resistance to cephalad opposition of the abdominal viscera when the patient is supine.
Relative contraindications to diaphragmatic plication include body mass index (BMI) greater than 35 due to technical difficulty and for the possibility that with aggressive weight loss/bariatric surgery the overall improvement of chest wall compliance may be sufficient to improve symptoms without surgical procedure. Patient with progressive neuromuscular disorders (amyotrophic lateral sclerosis or muscular dystrophy) are usually not candidates as is not only the diaphragm to be dysfunctional but are also the other accessory muscles of respiration. Other relative contraindications include bilateral hemidiaphragmatic elevation and a calcified, non-pliable diaphragm (4).
Surgery
There are two main techniques to manage diaphragmatic elevation due to eventration or paralysis in open surgery through a thoracotomy access: central imbrication technique and radial plication technique.
Central Imbrication Technique
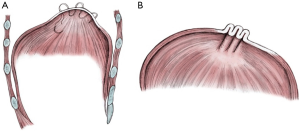
Under general anaesthesia with double lumen tube, an anterolateral thoracotomy is performed: the weakened diaphragmatic area is identified and lifted to determine the placement and the orientation of the suture lines. Linear rows of pledgeted nonabsorbable horizontal mattressed sutures (in our centre we use 2.0 Ethibond with Teflon pledgets) are placed through the weak part of the diaphragm (Figure 1A). It’s important to lift the diaphragm to avoid injury to the abdominal organs with the sutures. Sutures are then tightened to flatten the diaphragmatic surface (Figure 1B). Sutures placed within the central tendon will not be adequate to stabilize the diaphragm long term: several other sutures are required to be placed from the edge of the diaphragmatic tendon in an antero-posterior fashion to produce substantial caudal displacement of the tendon towards the abdominal cavity allowing expansion of the ipsilateral lower lobe as well as balancing the mediastinum
Radial plication technique
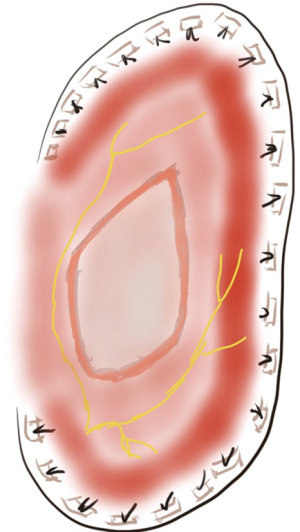
Firstly described by Dr. David State in 1949, this technique implies a repair performed with interrupted horizontal mattress pledgeted sutures imbricating the muscular portion of the diaphragm in a radial manner towards the chest wall via a posterolateral thoracotomy as an unbroken band from the xiphoid process to the vertebral body avoiding the mediastinal pleura (Figure 2). The efficacy of this approach lies upon vigorous displacement of the edge of the muscle-tendon interface towards the lateral chest wall (5). The sutures can anchor the endothoracic fascia inside the ribs or also pass around the ribs. If a first series of stitches has been placed without achieving the desired tautness of the flaccid diaphragm, a second row of stitches can be placed for further tightening of the muscle.
Postoperative care
Patients are usually extubated directly in theatre unless they already required mechanical ventilation as per preoperative period. A single 28F straight drain is left on suction at a pressure of −2 Kpa. A portable chest X-ray is obtained after surgery to assess the drain position, the lung expansion and the new baseline of the plicated hemidiaphragm. Chest drain is removed usually on day 1 postoperatively unless an air leak is present. Attention should be paid to any sign of peritonitis that could represent injury to the stomach or bowels.
Once managed these and other early stage complications, patients are usually ready to be discharged in 3 to 4 days.
Complications
Many different complications have been described in different case series. In general the spectrum of surgical complications are similar to that seen in pulmonary resections (atrial fibrillation, pleural effusion, pulmonary oedema, deep venous thrombosis, pulmonary embolism etc.) but with decreased risk of air leak or broncho-pleural fistula as the lung is not intentionally involved in the plication. Nevertheless there is a higher overall risk of abdominal organs injury (spleen, stomach, liver, colon and small intestine) which can occur while taking full thickness bites without proper retraction of the local tissue. Recurrent diaphragmatic elevation can be acutely caused by excessive coughing in the early postoperative period.
Conclusions
Diaphragmatic plication should be only performed on symptomatic patients with evidence of elevated diaphragm not caused by any other pathologic process other besides paralysis and eventration. Before surgery, patients must be evaluated for any other primary cause of dyspnea. PFT and chest radiograph are essential tools to evaluate the entity of the diaphragmatic elevation. CT scan is useful to rule out any other cause of phrenic nerve involvement. Different techniques have been described to manage paralysis and eventration via open or minimally invasive approach. The choice of plication approach is up to the surgeon’s expertise, training and preference. Improvement in dyspnoea is the most important measure of clinical success.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Shanghai Chest for the series “Open Thoracic Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.08.01). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. BB served as the unpaid Guest Editor of the series. MS served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor of Shanghai Chest. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deslauriers J. Eventration of the diaphragm. Chest Surg Clin N Am 1998;8:315-30. [PubMed]
- Ciccolella DE, Daly BD, Celli BR. Improved diaphragmatic function after surgical plication for unilateral diaphragmatic paralysis. Am Rev Respir Dis 1992;146:797-9. [Crossref] [PubMed]
- Graham DR, Kaplan D, Evans CC, et al. Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg 1990;49:248-51; discussion 252. [Crossref] [PubMed]
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg 2010;89:S2146-50. [Crossref] [PubMed]
- Mouroux J, Padovani B, Poirier NC, et al. Technique for the repair of diaphragmatic eventration. Ann Thorac Surg 1996;62:905-7. [Crossref] [PubMed]
Cite this article as: Patrini D, Panagiotopoulos N, Bedetti B, Lawrence D, Scarci M. Diaphragmatic plication for eventration or paralysis. Shanghai Chest 2017;1:25.


