
Reconstructive techniques after diaphragm resection and use of the diaphragmatic flap in thoracic surgery
Introduction
The diaphragm has been recognized as “the second most important muscle after the heart” (1).
Although a rare occurrence, the need for diaphragmatic resection and reconstruction might be dictated by secondary invasion from different tumours, including lung cancer, mesothelioma, chest wall tumours, sarcomas and metastatic lesions. Several factors must be taken into consideration when planning the approach to tumours involving the diaphragm, that accordingly to the histology, the amount and the site of involvement could be partially or completely removed. In some circumstances the resection might extend to adjacent structures such as the pericardium (mesothelioma) or the chest wall (sarcomas), thus posing supplementary challenges to the surgical procedure.
Some of the diaphragmatic resections can be repaired primarily, as long as there is adequate tissue that can be brought together without excessive tension. Larger defects or a completely resected diaphragm can be reconstructed only with synthetic or autologous tissue.
It should not be overlooked also that the diaphragm plays an important role as structure for plastic or reconstructive surgery and its use as vascularised flap in thoracic surgery has been widely documented by several reports for the treatment of multiple conditions (i.e., reinforcement of spontaneous or iatrogenic oesophageal perforations, repair after extensive pericardial resection, prophylactic bronchial stump coverage or early closure of bronchopleural fistula) (2).
Eventually large defects of one hemi-diaphragm might require reconstruction with prosthetic material in the context of a post-traumatic rupture with abdominal viscera herniation or for chronic symptomatic congenital hernia diagnosed late in adults.
Operative techniques
Surgical approaches to the diaphragm
The diaphragm provides the anatomical barrier between the pleural cavities/pericardium and the abdominal cavity. Its muscular nature is associated with the ability to sustain wide incisions without functional compromise even in the presence of bulky diseases or large defects. Surgical approach varies from classical trans-thoracic or trans-abdominal or combined thoraco-abdominal open approaches to minimally invasive thoracoscopic and laparoscopic approaches. It is impossible to establish precise criteria, surgical approaches depending on the nature of the lesion, its location, the existence of associated thoracic or abdominal abnormalities and eventually the surgeon’s attitude and preference. The surgical approach should be therefore individualized and based on the clinical and radiographic findings. Vast majority of the diseases where the diaphragm is involved and needs to be resected with are thoracic in their nature, thus requiring a thoracic approach.
In general, right-sided diseases are often difficult to repair from the abdomen because of the presence of the liver and hence a right thoracotomy is preferred. On both sides the thoracotomic approach ensures optimal access to all the muscular surface facilitating adequate surgical manoeuvring and treatment of any type of diaphragmatic lesion. Moreover, the thoracotomic approach is definitely indicated in case of chronic traumatic hernias where long-standings defects usually require prosthetic replacement and division of adhesions between abdominal and thoracic structures. The incision can be performed in the 6th or 7th intercostals space normally through a posterior muscle-sparing thoracotomy in the auscultatory triangle or a standard postero-lateral thoracotomy. Thoracoscopy and laparoscopy are very useful to explore both cavities in the case of undiagnosed or more complicated situation and can be used in conjunction to open approaches. Thoraco-abdominal combined approaches are rarely indicated and we prefer to repair the diaphragm through thoracic approach until and unless there is clear-cut indication for concomitant laparotomy (associated abdominal organ injury).
Reconstructive techniques after diaphragm resection
The techniques of reconstruction vary according to the underlying indication.
Primary repair
For brevity, in this chapter the direct reconstruction of the diaphragm will not be discussed in details as they do not require prosthetic re-shape usually because relatively small defects that can be secured with simple re-approximation of both the edges of the muscle.
It is, however, of crucial importance emphasize the need of tension-free remodelling with adequately large and strong filament material (#0, #1, #2 suture size) in order to prevent secondary disruption, especially on left side where the absence of the liver offers less protection and predispose to iatrogenic herniation of abdominal viscera. Care should be taken to take full-thickness bites, while avoiding injury to structures below the diaphragm. A variety of suture material and technique have been successfully employed: interrupted or horizontal mattress sutures, running suture repair or one-layer interrupted stitches closure are all commonly accepted techniques in current practice, both with absorbable or non-absorbable suture material (3).
We favoured in the last decade the Maxon MT-20 1 loop suture (Polyglyconate Monofilament Absorbable, Medtronic™, USA) because of its properties: high tensile strength, 180-days absorption profile, looped structure providing adjunctive tension and smooth-shaped needle minimizing tissue drag and trauma.
Total prosthetic diaphragmatic repair (mesothelioma, complex thoracic exenterations)
Reconstruction of the diaphragm in these context is performed through the surgical approaches preferred for the whole procedure usually a thoracotomy whether a lateral or poster lateral (extra-pleural pneumonectomy or pleurectomy/decortication for mesothelioma) or combined approach like hemi clamshell incision as in the case large thymomas or large chest wall tumours.
It is crucial to secure laterally with sutures the prosthetic patch around the ribs. Posteriorly, it is sutured to the diaphragmatic crus or gently tacked with finer sutures to the wall of the oesophagus. Medially it is sewn to the edge of the pericardium with interrupted sutures. Some Authors consider extremely important to place the diaphragmatic reconstruction at the same level as the native diaphragm, namely at the 10th intercostal space posteriorly and at the 8th–9th intercostals spaces anteriorly and laterally, in order to deliver adjuvant radiation safely, especially in the area of the posterior costo-phrenic sulcus, where the liver (right side) or the oesophagus and stomach (left side) might be exposed to an increased risk of radiation toxicity. However the modern technology with the use of Intensity-modulated radiation therapy (IMRT) technique enables a high-precision delivery, using computer-controlled linear accelerators to distribute precise radiation doses to specific areas with reduced side-effects (Figure 1).
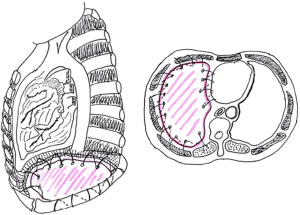
The experience says that usually the attitude is to over-correct the reconstruction placing the prosthesis in a lower position than the native diaphragm and with a more steep shape, thus the goal should be to place the anchoring stitches in the appropriate site still accepting a slightly more elevated position as the final result. This makes the reconstruction easier and avoids the need to insist on stitching in un-natural areas where the anchoring points result less safe.
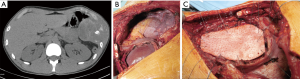
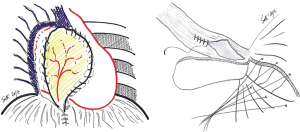
Specific consideration deserves the reconstruction on left site, because of the peculiar anatomy of the left posterior costo-phrenic corner. Not surprisingly the vast majority of diaphragmatic patch dehiscences reported in the literature occur on left side with an incidence as high as 5–7.6%. The special risk in this area is due to the absence of diaphragmatic tissue after the extensive resection of the whole muscle, leaving the left posterior costo-phrenic sulcus as the most critical site for diaphragmatic reconstruction especially for the proximity of the oesophageal hiatus and the descending aorta and no viable tissue that can be used to fix the stitches and patch. Sugarbaker et al. suggested to leave a 1- to 2-cm rim of left diaphragmatic crus over the gastric incisura where sutures can be placed during patch reconstruction preventing gastric herniation (Figure 2) (4).
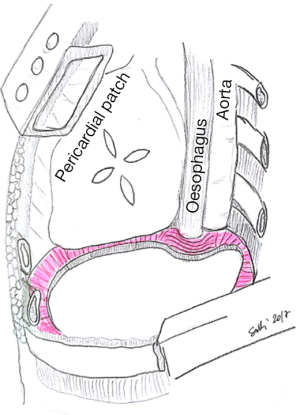
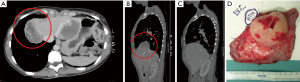
This is certainly a viable option although we believe that the technique described by Rea et al. (Figure 3) (5) with titanium plates support offers an easier and safer chance for diaphragmatic reconstruction. Titanium plates have characteristics of both endurance and flexibility, thus resulting in a valid and physiologic replacement, at the same time providing a good support for patch fixing thanks to multiple holes in its structure. Care must be taken to adequately expose the vertebral column and the posterior arch of the ribs (usually the 9th and 10th vertebra represent optimal fixing points) and to accurately identify site of screws insertion to avoid the potentially dangerous positioning into the intervertebral disc. Firstly a simulation by adapting an easy-flexible L-shaped plate model for fixation on two vertebral bodies and along the rib arch below should be done. The bone of the vertebral bodies is then exposed removing all the overlying tissue and the plate is fixed with two screws (14 mm length) on the two adjacent vertebral bodies and with one or two screws (12 mm length) on the corresponding rib. Finally, the patch is fixed to the plate with single stitches that are passed through the plate loops. The whole procedure takes about additionally 15 minutes (Figure 4).
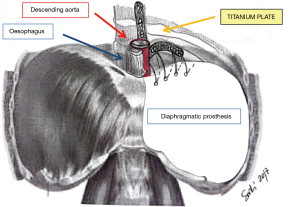
For analogue reasons reconstruction has to pay attention on right side: usually a notch of the mesh had to be patterned at the level of the inferior vena cava to avoid vena cava and hepatic veins constriction. However the presence of the liver makes the reconstruction safe even in the presence of a not completely medially reconstructed diaphragm and the incidence of herniation on right side is only anecdotic.
Partial prosthetic diaphragmatic repair
Bulky diseases (thymomas or other mediastinal malignancies) or huge chest wall tumours invariably require complete resection and reconstruction of the diaphragm. More challenging is how to decide for a direct reconstruction versus a prosthetic replacement in case of partial involvement of the muscle. Most frequent clinical scenario is a lung cancers infiltrating the diaphragm, occasionally limited diaphragmatic deposits (thymoma, ovarian cancers, sarcomas). In this context the phrenotomy is normally tailored in order to ensure an oncologically adequate clear margin. It could be realized circumferentially encircling the lesion or via a radial incision if a larger part of the muscle has to be resected or if the infiltration is in the periphery. It is crucial to progressively put stitches on diaphragmatic edges along with the opening of the abdominal cavity, in order to prevent muscular retraction and also to have at the end of the excision a clear delimitation of the defects and an optimal strategy to accurately plan the reconstruction (Figure 5).
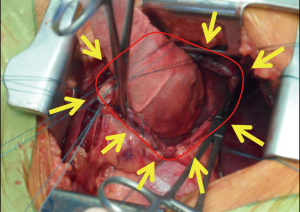
A specific consideration deserves the case of chronic traumatic diaphragmatic hernias in adult. These are usually long-standing conditions not rarely associated with relatively moderate diaphragmatic defects. Same principles apply and when the hernia is reduced in the abdominal cavity, the edges of the defect have to be delimited with multiple separated stitches to plan the reconstruction.
There is no a general rule on what is a defect “large enough” to require a prosthetic replacement and what could be directly sutured, depending on surgeon’s experience and situations. It should be considered the constant stress on the suture lines during respiration and the intra-abdominal pressure (again especially on left side) and also surgeons should remember that the real tension of the diaphragm is not that experienced during the procedure due to the anaesthetic management with curarization and muscles paralysis. In general only very limited defects can be safely repaired with direct suture and tension-free, for all the other conditions an even small prosthesis usually offers a more anatomical remodelling. The mesh is anchored through interrupted, tension-free stitches to the diaphragmatic remnants (Figures 6,7).
Diaphragm as vascularised flap
Diaphragmatic flaps have been successfully used to reinforce spontaneous and iatrogenic oesophageal perforations and to close chest wall defects for more than 30 years.
The diaphragmatic harvesting presents several advantages compared to other types of vascular pedicled flaps for intrathoracic transposition (i.e., serratus, intercostal, omental, pectoralis major, latissimus dorsi, parietal pleural and pericardial).
Firstly from the technical point of view: a customized flap is usually easy to prepare, to rotate, and to adapt to the purpose. Moreover it is thick enough, resistant to necrosis and infection, very well vascularised due to the extensive blood supply to the diaphragm. A long flap can easily be tailored and can reach any area of the thoracic cavity without torsion or tension. There is usually not need to change patient’s position on the operating table during the procedure, only occasionally a supplementary incision is required for harvesting.
The opening of the peritoneal cavity during phrenoplasty does not represent a real risk of propagating infection to the abdomen, nor functional disadvantages are caused by the phrenoplasty itself. As a minor functional effect after phrenoplasty, the motility of the posterior leaflet decreased with subsequent reduction of the costo-phrenic sinus. This may result in a benefit after pneumonectomy (reduction of the right post-pneumonectomy space) or after lobectomy en-bloc with diaphragm resection (shortening the period of air leakage, reduced basal space).
Moreover the closure of the diaphragm subsequent to flap harvesting decreases the muscle surface and reduces its paradoxical movement and may positively affect respiratory function improving the tolerance to pneumonectomy.
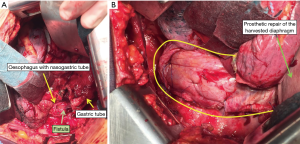
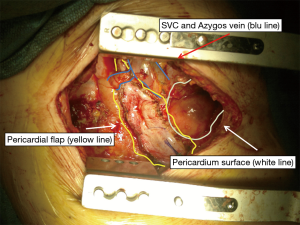
In this section the use of the diaphragm as flap is presented in pictures and photos in 4 different conditions: reinforcement of spontaneous or iatrogenic oesophageal perforations, repair after extensive pericardial resection (6), prophylactic bronchial stump coverage, and early closure of bronchopleural fistula (Figures 8-10).
Choice of prosthetic material
Many different materials (autologous or alloplastic) can be employed for the reconstruction, mostly favouring surgeon’s experience, attitude and preference.
In the pas there has been interest for the autologous reconstruction with muscle pedicled flaps (TRAM technique, external oblique muscle flap, autologous latissimus dorsi muscle reverse flap). Among these, the technique described by Bedini et al. (7) with the latissimus dorsi has been consistently employed during mesothelioma surgery or for large sarcomas with interesting results. Autologous flaps offer the advantage of vascularised tissue, without a permanent foreign body and the related potential infection risk. On the other hand, the harvesting technique is generally complex and time consuming and increases potential morbidity and donor-site complications.
Nowadays, the reconstruction is typically performed with synthetic meshes. They are well tolerated, can be bio-prosthetic materials or entirely artificial mesh, either absorbable or non-absorbable.
Because of its strength and impermeability, polytetrafluoroethylene (PTFE Gore-Tex™, Gore&Assoc, Arizona, USA) is one of the most common meshes recommended for diaphragmatic reconstruction after EPP or its composite variants (GORE® DUALMESH®) with dual-surface material that encourages host tissue in-growth while minimizing tissue attachment to abdominal viscera and optional antimicrobial technology. Other options include the non-absorbable and permeable prosthetic Mersilene polyester fiber mesh (Ethicon™, Somerville, NJ, USA), Prolene double-filamented polypropylene mesh (Ethicon™, Somerville, NJ, USA), Marlex polypropylene monofilamented fiber mesh (Davol™, Cranston, RI) even though rarely used in routine practice.
Less experience exists with new biological materials: bovine pericardium, acellular porcine dermal collagen (Permacol™ Covidien, AG, USA), acellular human cadaveric dermis (AlloDerm™, Lifecell Corporation, Branchburg, NJ, USA) or the composite mesh AlloDerm + polypropylene with the AlloDerm facing the abdomen. Biologic meshes use a newer technology where cells and immunogenic properties are removed, leaving behind only a basement membrane framework for native tissue cells to recolonize. Subsequently, implanted extracellular matrix will be degraded and host collagen deposited. Thus, the biologic mesh should allow the strength and integrity of the repair to be maintained while this remodeling occurs.
Very recently Rolli et al. reported impressive results with the Surgimesh-PET 3D (Aspide Medical, La Talauderie, France), a new alloplastic mesh made of knitted multifilament polyester with 3-dimensional structure; permeable to fluid and cell migration, chemically inert, non-absorbable, highly resistant, gives a stable framework for cell migration, allowing a definitive mesh incorporation and permanent internal support (8).
Comments: tips and tricks/caveats
- Primary repair of any diaphragmatic defect should be pursued only if the final reconstruction is tension-free with excellent re-approximation of edges. Prosthetic replacement should be considered in any other circumstances regardless the entity of the defect.
- Total diaphragmatic removal on right side at the level of the inferior vena cava (IVC) hiatus could be reconstructed with some tolerance because herniation is very rare due to the liver position; at the same time attention should be paid either during resection and reconstruction to the IVC itself and the sovrahepatic veins.
- Total diaphragmatic removal on left side on the contrary should be followed by meticulous and precise reconstruction; iatrogenic hernias are between the most common postoperative complication and are usually associated to technical mistakes in reconstruction (see text for details).
- When opening the diaphragm for a partial resection or during dissection for a chronic traumatic hernia, multiple stitches on the edges should be placed in advance in order to better plan the reconstruction and avoid abdominal viscera injuries.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.08.04). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. LB serves as an unpaid editorial board member of Shanghai Chest from Jun 2017 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fell SC. Surgical anatomy of the diaphragm and the phrenic nerve. Chest Surg Clin N Am 1998;8:281-94. [PubMed]
- Mineo TC, Ambrogi V. The diaphragmatic flap: a multiuse material in thoracic surgery. J Thorac Cardiovasc Surg 1999;118:1084-9. [Crossref] [PubMed]
- Finley DJ, Abu-Rustum NR, Chi DS, et al. Reconstructive techniques after diaphragm resection. Thorac Surg Clin 2009;19:531-5. [Crossref] [PubMed]
- DaSilva MC, Sugarbaker DJ. Technique of Extrapleural Pneumonectomy. Oper Tech Thorac Cardiovasc Surg 2010;15:282-93. [Crossref]
- Schiavon M, De Filippis A, Marulli G, et al. A new technique of diaphragmatic patch fixation in extrapleural pneumonectomy. Eur J Cardiothorac Surg 2010;38:798-800. [Crossref] [PubMed]
- Goldstraw P, Jiao X. Pericardial repair after extensive resection:v another use for the pedicle diaphragmatic flap. Ann Thorac Surg 1996;61:1112-4. [Crossref] [PubMed]
- Bedini AV, Andreani SM, Muscolino G. Latissimus dorsi reverse flap to substitute the diaphragm after extrapleural pneumonectomy. Ann Thorac Surg 2000;69:986-8. [Crossref] [PubMed]
- Rolli L, Leuzzi G, Girotti P, et al. Permeable Non-absorbable Mesh for Total Diaphragmatic Replacement in Extended Pneumonectomy. Ann Thorac Surg 2017;104:e105-7. [Crossref] [PubMed]
Cite this article as: Solli P, Bertolaccini L, Brandolini J, Pardolesi A. Reconstructive techniques after diaphragm resection and use of the diaphragmatic flap in thoracic surgery. Shanghai Chest 2017;1:21.


