Thoracic incisions for open surgery
Introduction
Despite the advances of minimally invasive techniques in the surgical management of thoracic diseases open surgery remains an important part of a surgeon’s activity. The extension of the disease both in size and location, the surgeon’s experience and the resources available will determine the decision to perform open surgery. Over the years, a variety of incisions to access the thoracic cavity have been described some with universal indications and others tailored to particular procedures. The principles remain the facility to perform the procedure safely and efficiently with adequate exposure to deal with the steps of the operation as well as the possible technical complications or difficulties. A surgeon, in order to decide the preferred incision, will take into account the own experience and training but also the indication for surgery, the anatomical location of the pathology, the likehood of encountering difficulties (such as previous surgeries or locally invasive tumours), the preoperative assessment of the patient, the predicted extent of side effects, and also the patients’ views in term of cosmesis and recovery.
In order for an incision to be effective we cannot forget some basic principles: careful positioning of the patient with clear identification of anatomical landmarks, correct handling of the tissues, step by step haemostasia, and gradual spread of the bony components (sternum or ribs) to minimise chances of inadvertent fractures. As important as the access is the closure of the incision, with correct suture by layers, haemostasia, and prevention of wound complication such as intercostal haernias, seromas or dehiscence. In this chapter, we will divide the incisions into three main groups: open approaches in lateral decubitus position (muscle and non-muscle sparing), open approaches in supine position (sternotomy, anterior thoracotomy, clamshell and their variations), and hybrid approaches (combination of open and minimally invasive). We will illustrate the main incisions with an interest to potential complications and pitfalls.
Open approaches in lateral decubitus
Lateral decubitus remains the most common position for open approaches to the thoracic cavity. It does allow exposure to the entire cavity, extension of the incision should be necessary, and the variability of anterior and posterior approaches. The positioning of the patient can vary slightly according to surgeons’ preferences, but the main principles are similar. Multiple pressure points should be adequately padded including a pillow between the legs and both arms flexed and without tension to avoid possible damage to the brachial plexus. The position of the non-dependent arm varies as per personal preferences and the location of the incision: flexed in a “prayer” position or elevated with a padded support. This can be more useful in anterior incisions. The patient must remain stable on the operating table to allow for table movements during the surgery. Stability can be achieved equally by beanbags, hard stops, or straps. The intercostal spaces should be widened by hyperextending the thorax either breaking the table or with the placement of a roll under the dependent chest at the level of the tip of the scapula. The surgeon will be placed anteriorly o posteriorly to the patient depending of the location of the incision.
Posterolateral thoracotomy
Posterolateral thoracotomy is the most traditional and used of the thoracic incisions. It is a multi-purpose approach as it will provide excellent exposure to mayor surgery, and it can be extended cranially such as the Poulsen approach or anteriorly if access to the diaphragm or even abdomen is needed.
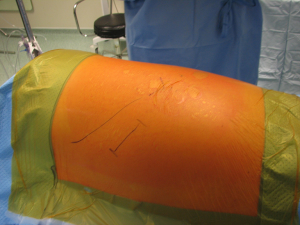
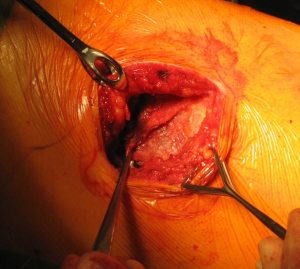
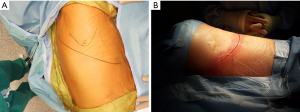
The anatomical markers will be a point 2–3 cm below the tip of the scapula, the mid-distance point between the middle of the spinal border of the scapula and the spinous process of the vertebra (Figure 1A). The skin incision will join these two points and it is extended anteriorly until the anterior axillary line (Figure 1B). After incision of the skin and subcutaneous layer (Figure 2), the latissimus dorsi muscle is carefully divided with cautery. Anteriorly the serratus anterior muscle was traditionally divided although nowadays is preserved by dividing its attachments to the ribs. Posteriorly the lateral border of the trapezius is exposed and can be divided in cases where more posterior access is required.
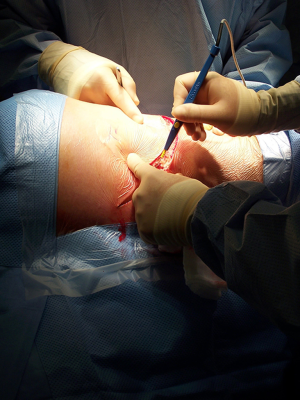
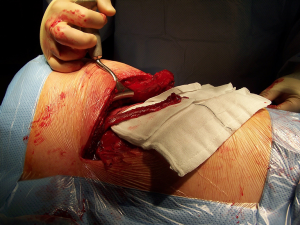
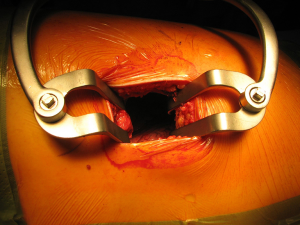
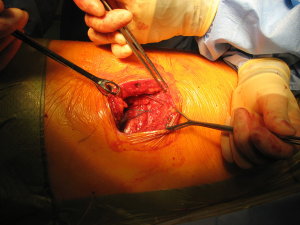
After the muscular layers are divided, the surgeon will enter the chosen intercostal space (Figure 3). This skin incision will allow deciding from 4th to 7th intercostal spaces without difficulties, and the spaces can be identified by counting the ribs posteriorly from the top. The intercostal space can be divided either directly by dividing the muscles or by lifting the periosteum off the rib below and should extend from costotransverse ligaments posteriorly to the internal thoracic vessels anteriorly (they must be preserved). If an intercostal muscle flap is thought to be necessary in cases of pneumonectomy or bronchoplasties, this flap must be raised at this point before placing the spreader (Figure 4). With the lung underneath collapsed the parietal pleura is entered. In cases that we expect adhesions it is advisable to mobilise some of the parietal pleura off the rib above and below the incision prior to enter the cavity to minimise the chances of accidental damage to the lung.
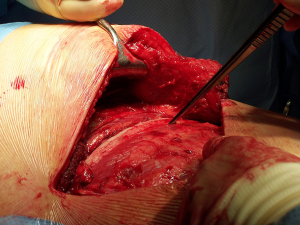
Once the parietal pleura is divided the rib spreader is inserted and should always be opened slowly and progressively, trying to avoid the possible risk of unwanted rib fractures. Manouvres to minimise this risk could be blunt division of the costo-transverse ligaments or even elective transection of the rib posteriorly, always with care to avoid damage to the intercostal vessels proximally. These vessels can retract into the spinal canal if divided accidentally and can be difficult to control.
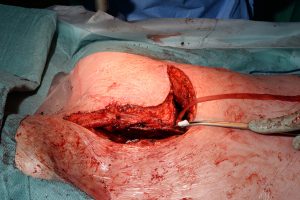
The traditional posterolateral thoracotomy allows the surgeon to perform the vast majority of thoracic procedures both in the elective and the emergency settings. Excellent exposure of the thoracic cavity and the familiarity with the position are the great advantages of this access. The surgeon is able to visualise and control most parts of the lung, thoracic aorta and oesophagus, diaphragm and pleura (Figure 5). It can also extended as mentioned before towards abdomen by converting into a thoraco-phrenotomy by dividing the diaphragm or even Thoraco-laparotomy by division of the costal margin; or towards the upper thoracic cavity by division of the posterior attachment of the scapula in cases of superior sulcus tumours or chest wall involvement.
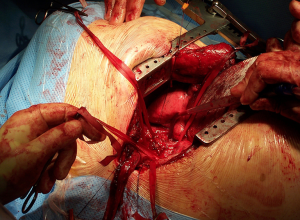
Once the procedure is finished, the intercostal spaces have to be adequately closed either by pericostal or intracostal sutures. Reducing the hyperextension of the chest by unbreaking the table or removing the roll will facilitate this step. Following this, the muscular layer should be closed with good haemostasia. Care must be put on opposing the ends of the Latissimus Dorsi adequately as when divided the caudal end will retract posteriorly and the cranial end anteriorly. Deficient closure of the muscle will lead to a persistent lump under the skin. Subcutaneous tissues and skin is then closed.
Posterolateral thoracotomy is a very traumatic procedure, carrying a risk for early and long term pain and an early impact on respiratory function following surgery. Division of the latissimus dorsi muscle can cause shoulder movement dysfunction that can delay return to full activities. Surgeons have gradually moved away of dividing serratus anterior or trapezius muscles and reducing the size of the skin incision, but it is still considered a posterolateral thoracotomy.
Muscle-sparing posterolateral thoracotomy
With the same patient position as the posterolateral approach a thoracotomy with preservation of the chest wall muscles can be performed via the Auscultatory triangle (1-7). The skin incision can be as large as required, but normally it is reduced to the initial part of the posterolateral thoracotomy incision without the need to extend the incision anteriorly to the tip of the scapula (Figure 6).
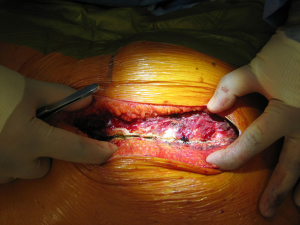
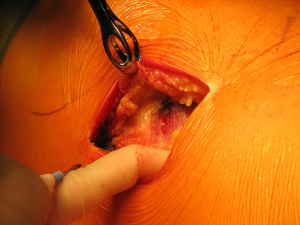
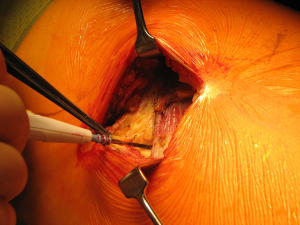
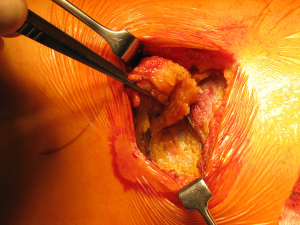
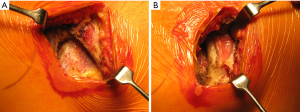
Once skin and subcutaneous tissues are divided (Figure 7), we identify the auscultatory triangle between the anterior edge of the Trapezius, the posterior edge of the Latissimus Dorsi, and the vertebral border of the scapula (Figure 8). Once identified the surface of the muscles, a subcutaneous flap is raised in all directions that will allow mobilising the muscles without dividing them. The fat and fascia between Latissimus and Trapezius is removed exposing the rib cage (Figure 9). The Latissimus musle is the retracted anteriorly and the Trapezius posteriorly as much as required depending of the procedure and exposure required. The intercostal muscle is entered in the same manner as in a posterolateral thoracotomy with the same precautions and the same length (Figure 10A,10B). The exposure is secured by the use of two smaller spreaders simultaneously, one retracting the ribs and another one at 90 degrees retracting the trapezius and latissimus muscles creating a square “window” (Figures 11,12).
One of the advantages of this approach is the speed of closure of the muscle layer once the intercostal space is closed (Figure 13). A single suture is run from the anterior Trapezius to the posterior edge of the Latissimus closing the entire layer with very easy haemostasia (Figure 14). Prior to closure of the skin it is important to place a tissue drain under the skin flaps to reduce the risk of seromas. This incision is very versatile as it can be potentially be as large as the posterolateral thoracotomy if required, and it is particularly helpful to treat pathologies of the posterior mediastinum (neurogenic tumours, oesophageal cysts…), which don’t require ample exposure to the anterior chest cavity. In terms of lung resections, we have found it extremely useful for anatomical segmentectomies of segment 6, procedures that involve reconstruction of the bronchial tree such as sleeve lobectomies or trauma because of their posterior location, and as an initial procedure for pulmonary metastasis as the incision would allow inspection of the entire lung, but it might minimise the adhesions should further procedures are required in the future.
Cosmetically it is an advantage to larger skin incisions and probably reduces the incidence of shoulder problems. Preserving the Latissimus Dorsi and Serratus muscles intact does allow for these muscles to be used in the future as flaps if complications or further procedures are required (4,8). Some of the difficulties with this incision are: the access to the main pulmonary artery in very central tumours can be more complex, there is a reported incidence of seromas of up to 26% so it requires the placement of a self-vacuum subcutaneous drain, and sometimes knot tying can be difficult as well as some angulation required in surgical staplers
A good tip is to create an anterior chest drain port early during the procedure and use it for insertion of the stapler guns, giving a very comfortable angle for placement and firing. It is an excellent incision when hybrid (VATS and limited open) access is planned.
Muscle-sparing antero-lateral thoracotomy
The patient is positioned in lateral decubitus with the same precautions as the posterolateral thoracotomy. The table can be slightly tilted posteriorly to facilitate surgeon’s view (who will be standing in the front of the patient). The skin incision is performed starting from the posterior axillary line in front of the tip of the scapula towards the submammary crease. The subcutaneous layers are divided with cautery, it is important to preserve the intercostal brachial nerve that runs in the superior part of the wound. If dissected a postoperative numbness of the lateral breast and the nipple area can occur. Posteriorly the long thoracic nerve can be identified and should be preserved.
The anterior border of the latissimus dorsi is mobilized posteriorly by creating a subcutaneous flap and it is spared. The lateral edges of the Pectoralis muscles are preserved without difficulties. The 4th or 5th intercostal space is entered in the same manner as per an anterior thoracotomy depending of the procedure. While it is more difficult to divide the costo-transverse ligaments to increase rib spreading, the intercostal spaces are wider anteriorly thus facilitating exposure.
The closure will be very similar to the procedure via auscultatory triangle, with the very important subcutaneous drain to prevent seromas or haematomas.
The wide anterior intercostal spaces facilitate exposure compared to the more posterior approaches thus in theory reducing the need for excess rib spreading. It gives a very adequate exposure of lungs, pericardium and diaphragm. Preservation of the chest wall muscles not only reduces impact on shoulder movements, but once again, allows for their potential use as flaps should complications (bronchopleural fistula) arise (4,8).
With the increase in the use of the VATS anterior approach the view is now very familiar for younger Thoracic Surgeons who recognise promptly this view for access to hilar structures even in central tumours. This might help for this incision to become the preferred “open” approach in the future and a good alternative for conversions from VATS to open surgery.
Some procedures might be more difficult, although not impossible, to perform with this approach. If we have to perform a broncho-angioplasty in the right side it will be easier to perform the bronchial reconstruction first (as it is located more posterior) and then the vascular one. For posterior anatomical segmentectomies (segments 3 or lateral basal), this access may prove more difficult than using more posterior approaches. The excellent access to hilar structures makes it extremely helpful for vascular control in central tumours.
Axillary thoracotomy
Axillary thoracotomy is less used than other “lateral” approaches. The lateral decubitus position is slightly altered by tilting the table backwards increasing exposure of anterior chest wall and the ipsilateral arm is secured away from the incision by shoulder abduction and elbow flexion at 90 degrees. As always when the arm is secured, care must be taken for it not to be in tension that might result in brachial plexus traction injury.
The axillary approach can be used for access to the high intercostal spaces (1st) down to 4th or 5th space depending of the indications for surgery. With the patient correctly positioned a transverse incision is performed at the axillary hair-line between the anterior axillary line (posterior edge of Pectoralis Minor) and posterior axillary line (anterior edge of Latissimus Dorsi), which are well demarcated by the bulk of the muscles. The creation of subcutaneous flaps to allow retraction of the latissimus dorsi posteriorly and pectoralis major anteriorly can extend the exposure for more extensive procedures. The digitations of the serratus anterior muscles can either be detached or divided to increase exposure. The ribs are then exposed and entry into the desired intercostal space is then performed according to surgeon’s preference.
The development of VATS techniques has reduced the use of these incisions. Traditionally surgeons have used them as an access for apical procedures: surgery for pneumothorax, sympathectomies and operations at the thoracic outlet (9-12). As a muscle-sparing thoracotomy, it is well tolerated and has got cosmetic results as the incision will remain in a non-exposed area. The exposure can be limited specially in the lower hemithorax.
If the procedure is complex the incision could be extended but it can be quite morbid in the more apical procedures. At the posterior end the long thoracic nerve should be preserved while dividing the serratus anterior. Due to the creation of the flaps this incision carries the risk of seromas and haematomas so care must be taken during closure in terms of haemostasia, approximating the ribs and closure of the serratus anterior. The incision is placed in quite a sensitive area liable to skin discomfort.
Open approaches in the supine position
Median sternotomy
Median sternotomy is the incision most commonly used for cardiac surgical procedures. It is also a very useful access to the thorax for different pathologies and anatomical locations.
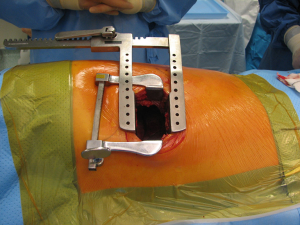
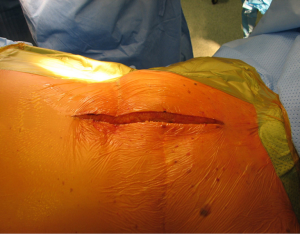
With the patient in the supine position with slight neck extension a longitudinal skin incision is performed in the midline from the sternal notch to the tip of the manubrium followed by dissection of the subcutaneous layers and pectoralis fascia (Figures 15,16). Once the midline of the sternum is identified accurately to avoid paramedian split of the bone the periosteum is scored with diathermy (Figure 17). By retracting the upper end of the incision, the space of Burns is explored, the interclavicular ligament is divided with care of not to damage the underlying inmoninate vein. Following that the xyphoid process is dissected at the bottom end of the incision. Some surgeons elect to blunt dissect the ends of the retrosternal space before splitting the sternum alongside with a saw (Figure 18). Haemostasia must be ensured at the periosteum and bone marrow with cautery and/or bone wax prior to spread of the incision by a retractor which must be done gradually.
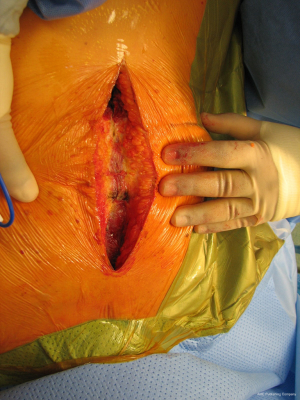
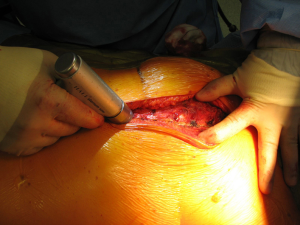
With this procedure, there is ample exposure to perform surgical procedures such as excision of pathologies in the anterior mediastinum, or by opening the pleurae to access either thoracic cavity (Figure 19A,19B). A particular use of median sternotomy is the access to the carina by entering the pericardium and later on the posterior pericardium. At the end of the procedure the sternal ends are approximated with sternal wires, 2 in the manubrium and 4 in the body, although it depends on patient’s height (Figure 20). A good closure of the sternum is important to minimize the risk of non-union, mediastinitis and wound infection. The pectoralis fascia, the subcutaneous layer and skin are then carefully closed by layers.
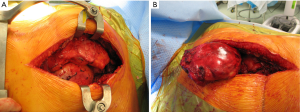
In non-cardiac thoracic surgery, median sternotomy has been extensively used in the management of large anterior mediastinal masses, maximal thymectomy for myasthenia gravis and bilateral pathologies such as multiple lung metastases. Median sternotomy was also the incision of choice following the resurgence of Lung Volume Reduction Surgery in the 1990s although more recently bilateral VATS (even as staged procedures) is more commonly used as it might reduce morbidity (13-18).
But median sternotomy can also be used in occasions for surgery of pleuropulmonary diseases specially on the right side when the surgeon can predict difficulties in exposing the main pulmonary vessels, such as central primary cancers in which pericardial involvement is suspected, or re-do procedures such as completion pneumonectomies for central tumours. The authors found out that right extrapleural pneumonectomies for mesothelioma were feasible via median sternotomy and indeed reduced the incidence of postoperative complications.
The advantages are a great exposure of mediastinal structures (even carina), easy access to control main pulmonary vessels, and ability of entering both pleural cavities. From the experience in the postoperative management and analgesia requirements following cardiac surgery we learnt that a median sternotomy is very well tolerated by patients with lesser incidence of pain related complications in the short and the long term than a large posterolateral thoracotomy.
Infective and sternal complications are uncommon (less than 5%) but potentially very morbid (19,20). Unfortunately, median sternotomy provides poor exposure to the posterior areas of the lung and mediastinum, as well as a very difficult access to the left lower lobe due to the position of the heart.
Thoracosternotomy (clamshell and hemiclamshell)
The Clamshell incision of a bilateral anterior thoracotomy with transverse sternotomy has been the choice for bilateral lung transplantation and access to pericardium in the past. Today ituse is restricted to very uncommon indications but remains a valuable tool mainly in trauma settings (21-25). A unilateral approach such as the hemiclamshell has also been described for diverse indications (26).
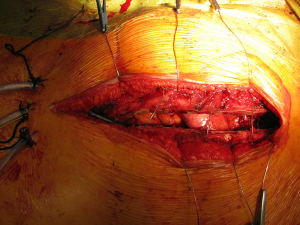
With the patient in the supine position the skin incision is made along the inframammary skin creases from the midaxillary/anterior axillary lines. The subcutaneous tissues are divided with cautery and the pectoralis major muscles are raised from their inferior and sternal attachments. The 4th or 5th intercostal spaces are identified and entered bilaterally. The internal mammary vessels are identified and ligated. The sternal body is divided transversally with a saw and after haemostasia the ends are spread with two sternal retractors (Figure 21). In the hemiclamshell approach the skin incision is performed starting on the sternal notch on the midline and running over the inframammary crease towards the anterior axillary line. The pectoralis major is raised or incised over the 5th rib. The 4th intercostal space is entered. The mammary bundle is equally ligated. Variants of this incision include a cervical extension of the incision along the medial edge of the sternocleidomastoid muscle with sternal splitting (Grunenwald’s, Figures 22-25), or the Dartevelle’s approach that include the sectioning of the medial half of the clavicle. These variants are extremely useful for excision of Superior Sulcus tumours and the hemiclamshell tends to be performed at higher intercostal spaces.
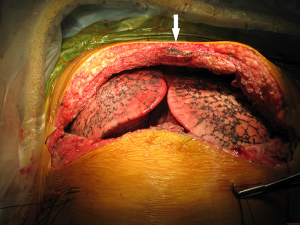
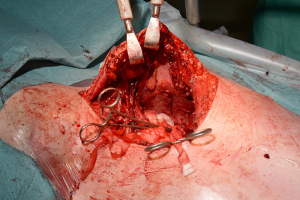
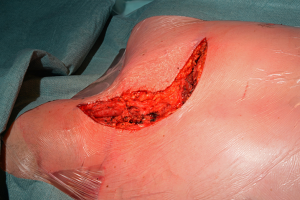
For closure, the sternal ends are approximated with wires, the intercostal spaces are closed routinely and the pectoralis major muscles are reattached to lower ribs and sternum (Figure 26).
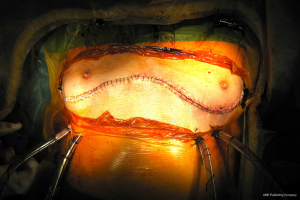
The clamshell incision provides the maximal exposure of thoracic cavities (Figure 27), but its comorbidities and the trauma involved have restricted its use to specific indications. Currently its use is limited to very large mediastinal masses (Figure 28), bilateral lung transplantation and trauma. Its use on bilateral lung metastases or excision of pericardium has declined in favour of other approaches.
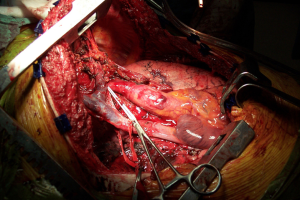
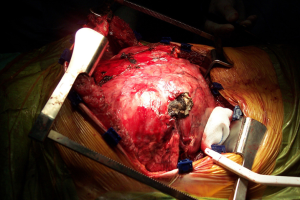
The rationale for the restriction in its use is mainly due to its consequences. Transternal division carries a high risk of sternal healing complications of up to 30% compared to 1-2% after median sternotomy. In a comparative series, Machiarinni et al reported more postoperative pain, deformities, need for surgical revision and more impact on respiratory mechanics following clamshell than median sternotomy after double lung or heart-lung transplant (25).
Anterior thoracotomies
The anterior thoracotomy did regain some popularity with the advent of Minimal Invasive Cardiac Surgery (mitral valve surgery and MIDCAB) (27), although its use in thoracic surgery remains limited to non-complex procedures.
The patient is positioned supine with a roll under the ipsilateral side of the operation to increase intercostal spaces separation. The skin incision is made from the anterior axillary line curving under the breast towards the sternum for procedures that require access through the 4th or 5th intercostal space. A lower approach of an anterior thoracotomy would be a submammary incision that can be very useful for insertion of pacemakers or creation of pericardial windows. In procedures for staging of lung cancer the incision is placed at the level of the 2nd-3rd intercostal space with or without excision of the sternocostal cartilage.
A limited anterior thoracotomy is a good approach for patients that require a lung biopsy but is not fit enough for a single lung ventilation that could make VATS approach more difficult, and it can be used as invasive mediastinal staging of lung cancer in the form of an anterior mediastinotomy for biopsy of lymph nodes or to confirm suspected direct mediastinal invasion. A good advantage is the fact that the intercostal spaces are wider anteriorly so less rib retraction is required, even being substituted by soft tissue retractors.
In the field of more extensive thoracic procedures, pure anterior thoracotomies provide a restricted exposure hence it is rarely used (28). If the costal cartilage is excised there is a risk of seromas or intercostal haernias, and the possibility of damage to the internal thoracic arteries.
Hybrid approaches
The application of VATS to assist open surgery has been used by surgeon since the advent of minimally invasive techniques. It can help reduce size of the incision and increase view in some areas (29). Authors have described the use of hybrid procedures in lung tumours involving the chest wall by performing the pulmonary resection through VATS and then the chest wall resection and reconstruction via a limited open approach even on superior sulcus tumours (30-32). The authors reported the use of single port VATS for the confirmation and “mapping” of the chest wall involvement thus allowing correct placement of the incision with limitation of trauma to the tissues (33) (Figures 29-32). Another use of the hybrid techniques is the initial assessment of patients requiring open re-do surgery where dense adhesions are expected and “blind” redo thoracotomy could lead to damage to pulmonary parenchyma. With the help of a single port VATS (whose port will be used for the intercostal drain placement) we can identify the safe area to perform the open incision and even divide adhesions under direct optical vision. New hybrid approaches have also been described for mediastinal surgery (34).

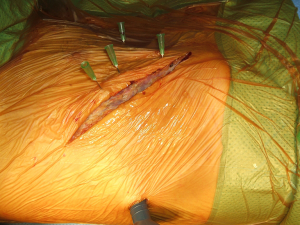
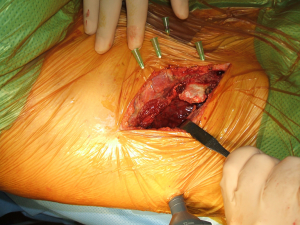
Summary
The access to the thoracic cavity during surgery is open to many incisions and approaches. The surgeons’ experience and training, adequate planning and the indications and anatomical locations of the surgery will influence the choice of approach. All the incisions have advantages and limitations, and it is important to be familiar with the different possibilities. All these incisions should be part of the surgeons’ arsenal of choices in order to individualise patients’ care providing adequate exposure with the minimal trauma. It is important to be aware of the specific tips regarding identification and preservation of vascular and neural structures to minimise adverse consequences. Equally important is a correct closure of the different planes and prevention of seromas and haernias.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.11). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mitchell R, Angel W, Wuerflein R, et al. Simplified lateral chest incision for most thoracotomies other than sternotomy. Ann Thorac Surg 1976;22:284-6.
- Bethencourt DM, Holmes EC. Muscle-sparing posterolateral thoracotomy. Ann Thorac Surg 1988;45:337-9. [Crossref] [PubMed]
- Horowitz MD, Ancalmo N, Ochsner JL. Thoracotomy through the ausculatory triangle. Ann Thorac Surg 1989;47:782-3. [Crossref] [PubMed]
- Ziyade S, Baskent A, Tanju S, et al. Isokinetic muscle strength after thoracotomy: standard vs. muscle-sparing posterolateral thoracotomy. Thorac Cardiovasc Surg 2010;58:295-8. [Crossref] [PubMed]
- Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg 1991;101:394-400; discussion 400-1. [PubMed]
- Athanassiadi K, Kakaris S, Theakos N, et al. Muscle-sparing versus posterolateral thoracotomy: a prospective study. Eur J Cardiothorac Surg 2007;31:496-9; discussion 499-500. [Crossref] [PubMed]
- Nazarian J, Down G, Lau OJ. Pleurectomy through the triangle of auscultation for treatment of recurrent pneumothorax in younger patients. Arch Surg 1988;123:113-4. [Crossref] [PubMed]
- Li S, Feng Z, Wu L, et al. Analysis of 11 Trials Comparing Muscle-Sparing with Posterolateral Thoracotomy. Thorac Cardiovasc Surg 2014;62:344-52. [Crossref] [PubMed]
- Becker RM, Munro DD. Transaxillary minithoracotomy: the optimal approach for certain pulmonary and mediastinal lesions. Ann Thorac Surg 1976;22:254-9. [Crossref] [PubMed]
- Ochroch EA, Gottschalk A, Augoustides JG, et al. Pain and physical function are similar following axillary, muscle-sparing vs posterolateral thoracotomy. Chest 2005;128:2664-70. [Crossref] [PubMed]
- Freixinet JL, Canalís E, Juliá G, et al. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg 2004;78:417-20. [Crossref] [PubMed]
- Han S, Yildirim E, Dural K, et al. Transaxillary approach in thoracic outlet syndrome: the importance of resection of the first-rib. Eur J Cardiothorac Surg 2003;24:428-33. [Crossref] [PubMed]
- Meng RL, Jensik RJ, Kittle CF, et al. Median sternotomy for synchronous bilateral pulmonary operations. J Thorac Cardiovasc Surg 1980;80:1-7. [PubMed]
- Urschel HC Jr, Razzuk MA. Median sternotomy as a standard approach for pulmonary resection. Ann Thorac Surg 1986;41:130-4. [Crossref] [PubMed]
- Martin-Ucar AE, Stewart DJ, West KJ, et al. A median sternotomy approach to right extrapleural pneumonectomy for mesothelioma. Ann Thorac Surg 2005;80:1143-5. [Crossref] [PubMed]
- Edwards JG, Martin-Ucar AE, Stewart DJ, Waller DA. Right extrapleural pneumonectomy for malignant mesothelioma via median sternotomy or thoracotomy? Short- and long-term results. Eur J Cardiothorac Surg 2007;31:759-64. [Crossref] [PubMed]
- Asaph JW, Handy JR Jr, Grunkemeier GL, et al. Median sternotomy versus thoracotomy to resect primary lung cancer: analysis of 815 cases. Ann Thorac Surg 2000;70:373-9. [Crossref] [PubMed]
- Welti H. Upper median sternotomy in the treatment of mediastinal tumors; 7 personal cases. Mem Acad Chir (Paris) 1950;76:638-54. [PubMed]
- Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs superficial infection. Chest 1996;110:1173-8. [Crossref] [PubMed]
- Robicsek F, Daugherty HK, Cook JW. The prevention and treatment of sternum separation following open-heart surgery. J Thorac Cardiovasc Surg 1977;73:267-8. [PubMed]
- Bains MS, Ginsberg RJ, Jones WG 2nd, et al. The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor. Ann Thorac Surg 1994;58:30-2; discussion 33. [Crossref] [PubMed]
- Germain A, Monod R. Bilateral transversal anterior thoracotomy with sternotomy; indications and technics. J Chir (Paris) 1956;72:593-611. [PubMed]
- Bains MS, Ginsberg RJ, Jones WG 2nd, et al. The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor. Ann Thorac Surg 1994;58:30-2; discussion 33. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Sood S, et al. Resection of primary mediastinal non-seminomatous germ cell tumors: a 28-year experience at memorial sloan-kettering cancer center. J Thorac Oncol 2011;6:1236-41. [Crossref] [PubMed]
- Macchiarini P, Ladurie FL, Cerrina J, et al. Clamshell or sternotomy for double lung or heart-lung transplantation? Eur J Cardiothorac Surg 1999;15:333-9. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [PubMed]
- Lucà F, van Garsse L, Rao CM, et al. Minimally invasive mitral valve surgery: a systematic review. Minim Invasive Surg 2013;2013:179569
- Schuchert MJ, Souza AP, Abbas G, et al. Extended Chamberlain minithoracotomy: a safe and versatile approach for difficult lung resections. Ann Thorac Surg 2012;93:1641-5; discussion 1646. [Crossref] [PubMed]
- Giudicelli R, Thomas P, Lonjon T, et al. Video-assisted minithoracotomy versus muscle-sparing thoracotomy for performing lobectomy. Ann Thorac Surg 1994;58:712-7; discussion 717-8. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Demmy TL, Nwogu CE, Yendamuri S. Thoracoscopic chest wall resection: what is its role? Ann Thorac Surg 2010;89:S2142-5. [Crossref] [PubMed]
- Shikuma K, Miyahara R, Osako T. Transmanubrial approach combined with video-assisted approach for superior sulcus tumors. Ann Thorac Surg 2012;94:e29-30. [Crossref] [PubMed]
- Bayarri CI, de Guevara AC, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Zieliński M, Kuzdzał J, Szlubowski A, et al. Transcervical-subxiphoid-videothoracoscopic "maximal" thymectomy--operative technique and early results. Ann Thorac Surg 2004;78:404-9; discussion 409-10. [Crossref] [PubMed]
Cite this article as: Martin-Ucar AE, Socci L. Thoracic incisions for open surgery. Shanghai Chest 2017;1:20.