
Right pneumonectomy
Indications
A right pneumonectomy most commonly is performed to treat lung cancer that is centrally located (1). Other indications for malignancy involve multiple lung metastases that cannot be resected using parenchymal sparing techniques and rare vascular tumors arising from the pulmonary artery or veins. Pneumonectomy is occasionally indicated for benign conditions, such as bronchiectasis and chronic infection resulting in a destroyed lung that is associated with respiratory symptoms and poses a risk of infecting the remaining good lung. We have performed a right pneumonectomy on rare occasions for recurrent massive hemoptysis without a localized source and after having exhausted interventional management options.
In patients in whom cancer is suspected, it is critical to obtain histologic confirmation, make a physiologic assessment of resectability, and rule out the existence of distant metastases. Bronchoscopy, chest and upper abdomen computed tomography (CT) with intravenous contrast, positron emission tomography (PET), and endobronchial ultrasound or mediastinoscopy for nodal assessment are generally required.
All candidates for a pneumonectomy should undergo a preoperative cardiopulmonary function evaluation. This assessment includes a complete history and physical exam, and measurement of spirometry and diffusing capacity. Additionally, in patients with borderline function, quantitative perfusion (V/Q) scan and measurement of maximum oxygen consumption during exercise (peak VO2) are indicated.
Operative technique
Preparation
A double lumen endotracheal tube is preferred for airway management and right lung isolation. Place the patient in the left lateral decubitus position. The type and location of the incision must be decided based on the indication for surgery, the amount of exposure needed, and the physical characteristics of the patients. We prefer to use a lateral muscle sparing thoracotomy in order to maintain shoulder girdle function, decrease postoperative pain, and preserve chest wall muscles for treatment of potential airway complications. Nevertheless, a wider field of exposure is sometimes necessary if the patient has a large central tumor making access to the hilum difficult, proximal airway involvement, or if challenging vascular dissections are encountered. Usually, the chest is accessed through the fifth intercostal space. However, in obese patients with high diaphragms, the fourth intercostal space is preferred.
Exposition
Chemoprophylaxis for venous thromboembolism is used routinely perioperatively. Before beginning the dissection, explore the chest cavity. A full assessment of the lung, hilar vessels, and nodes is performed to ensure that an R0 resection is feasible. The pulmonary ligament is divided. A complete mediastinal nodal dissection is performed, including stations R9, R8, 7 and R4. Suspicious nodes are assessed with frozen section if the identification of N2 nodal involvement would alter the surgical plan.
Operation
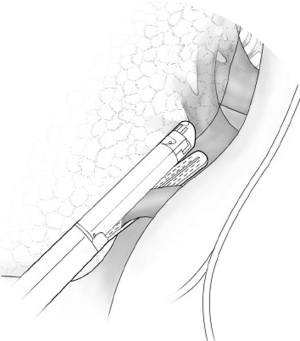
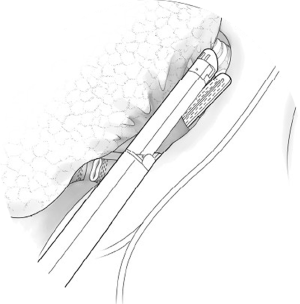
Begin by dividing the pleura surrounding the hilum. Both pulmonary veins are dissected circumferentially (Figure 1). After ascertaining that the pneumonectomy can be completed safely, these vessels are divided with a linear cutting stapler or with sequential application of an anvil-type stapler (Figures 2,3). The right pulmonary artery is dissected circumferentially to the pericardial reflection (Figure 4). The artery is divided with sequential application of anvil-type staplers (Figure 5) or a linear stapler, or may be clamped, divided, and oversewn if application of staplers is technically difficult (Figure 6).
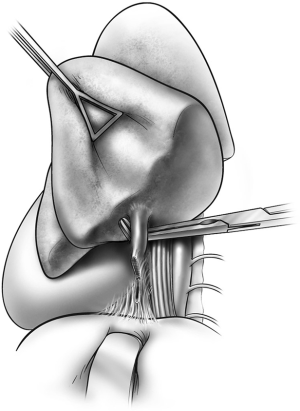
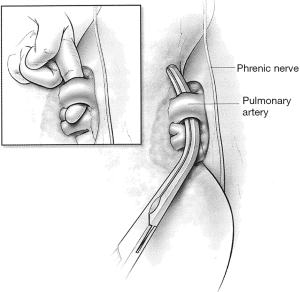
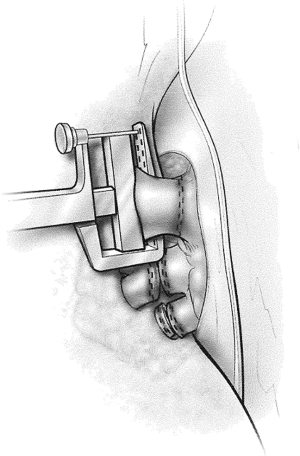
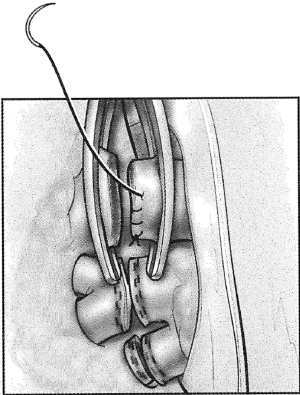
If dissection of one of these three vessels is likely to be difficult, the other two vessels are dissected first and then encircled with a vessel loop and gently snared. If injury to the third vessel occurs, bleeding can be greatly reduced by closing the snares to occlude the other two vessels. Injuries to the right pulmonary artery are challenging to control because there is very little room available to place an occluding vascular clamp. An intrapericardial dissection between the superior vena cava and the aorta allows cross clamping of the right pulmonary artery at its take off from the pulmonary trunk (Figure 7). In other circumstances, inflow occlusion obtained by snaring down both vena cava may provide a few minutes of bleeding control and sufficient exposure to repair the pulmonary artery injury.
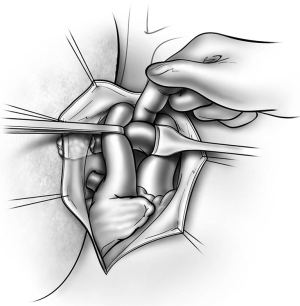
The bronchus is dissected circumferentially at its origin, taking care not to devascularize the trachea or left main bronchus. An adequate dissection of the subcarinal space and sometimes resection of the azygos arch are necessary to provide better exposure to the origin of the right main bronchus. The right main bronchus is then closed with a linear non-cutting stapler, occluded distally with a bronchial clamp and divided (Figure 8). An appropriate staple height commensurate with the thickness of the bronchus should be selected to ensure an airtight closure. When the tumor is close to the carina, the bronchus is divided with a knife at the carina without clamping the airway. The bronchus is then closed with interrupted absorbable sutures, taking care to avoid damaging the bronchial balloon of the double lumen endotracheal tube.
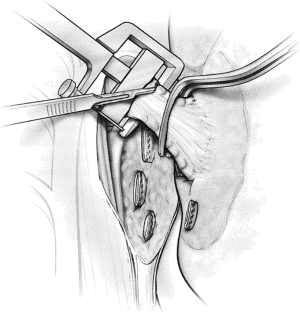
The risk of stump leak can be reduced by covering the stump with a flap of vascularized tissue. One option is to raise a pleural flap lateral to the azygos based superiorly (Figure 9). The flap must be sufficiently wide to be sutured to viable soft tissue at the medial extent of the bronchial dissection and sufficiently long to reach over the azygos vein to cover the stump without tension. Interrupted sutures should be used to tailor the pleural flap coverage over the bronchial stump. Other options for tissue flaps include intercostal muscle, pericardial fat (Figure 10), pericardium, the azygos vein, and a muscle flap such as latissimus dorsi or serratus anterior transposed into the chest.
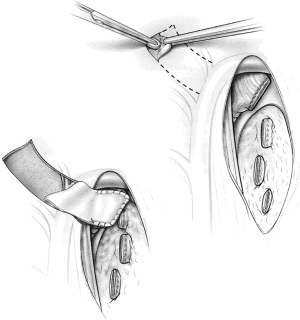
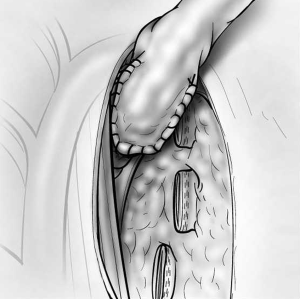
The pericardium must be closed after a right intrapericardial pneumonectomy to avoid cardiac herniation. Primary closure is sometimes possible, but the use of a patch to eliminate the pericardial defect has advantages. A pneumonectomy, particularly on the right side, is associated with significant right heart dysfunction and right ventricular dilatation. Primary pericardial closure reduces the size of the pericardial space, restricting cardiac function. The effects of this are usually negligible in healthy people, but in the postpneumonectomy patient, this can lead to restrictive effects that become evident 24 to 48 hours postoperatively and are poorly tolerated. Use of a small fenestrated patch, such as expanded polytetrafluoroethylene (PTFE) that is sutured to the pericardium edges, prevents cardiac herniation and eliminates the risks of pericardial restrictive effects in the immediate postoperative period (Figure 11).
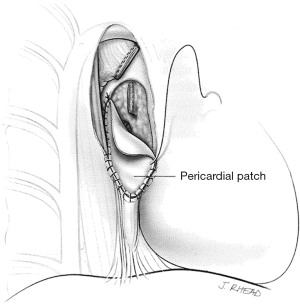
Meticulous hemostasis should be obtained because postoperatively there is no longer a lung to tamponade minor sources of bleeding.
There is controversy as to whether a pleural drain should be used postoperatively. One advantage of the drain is that it may be used after the thoracotomy incision is closed to balance the mediastinum in the center of the chest. This is done by first positioning the patient, who is still intubated and sedated, supine on the operating table. The pleural drainage system is then placed at the level of the right heart and on water seal. The contralateral lung is inflated and maintained at a constant airway pressure of 40 cmH2O for 5 to 10 seconds. After excess air is evacuated from the pneumonectomy space during the positive pressure inspiration, the drainage tube is clamped before the inspiratory cycle is terminated. The drainage tube is then left clamped unless a large hemothorax or hydrothorax develops, which is relieved by temporarily unclamping the chest tube. The potential down side of placing a chest tube is that there is an increased risk of contaminating the pleural space, leading to postpneumonectomy empyema. If a tube is used, it should be connected to a balanced drainage system, which prevents the development of excessive negative or positive intrathoracic pressure that can lead to mediastinal shift with its attendant cardiovascular consequences or to volotrauma of the remaining lung.
An alternative method of balancing the mediastinum when a drain is not left in place is to position a soft rubber catheter through the incision as it is being closed, weaving the catheter gently through the layers as they are sutured so that the tube does not lie in a straight tract. Before the skin is closed but after an airtight seal is achieved, the tube is placed under water seal and the patient is given a sustained breath as previously described. Once excess air has been evacuated, the tube is quickly withdrawn and normal ventilation is resumed. The remainder of the incision is then closed.
If the mediastinum has not been adequately medialized with these techniques based on postoperative chest radiograph, and if there is hemodynamic compromise, the mediastinum should be repositioned while the patients is in the postanesthesia care area. A large bore angiocath attached to a stopcock is inserted in the fourth intercostal space in the midclavicular line under sterile conditions, and an appropriate volume of air (usually 500 to 1,000 mL) is added or removed as is necessary to achieve the desired result.
Completion
Analgesia after pneumonectomy is crucial to prevent respiratory complications, allow early mobilization from bed to chair, and facilitate early ambulation. Multimodality analgesia is usually necessary in the early postoperative period, which includes oral or intravenous narcotics and nonsteroidal anti-inflammatory drugs (NSAIDs), intercostal blocks with local anesthetics, and epidural infusion of narcotics and local anesthetics.
Clearance of respiratory secretions and re-expansion of the atelectatic contralateral lung are facilitated by early and frequent use of incentive spirometry. Intravenous fluid administration is restricted to avoid fluid overload and pulmonary edema. Oral alimentation is resumed on the same day of surgery after recovery from anesthesia.
Chemical prophylaxis against atrial fibrillation is routinely used beginning in the immediate postoperative period.
Patients are discharged after the pleural drain is removed if they are ambulating independently, if pain control with oral analgesics is adequate, and when vital signs are close to normal. All patients are discharged on pharmacological prophylaxis for atrial fibrillation. If the operation was performed for cancer, patients are discharged on pharmacological thromboembolic prophylaxis for four weeks. Ambulation and frequent use of the incentive spirometer after discharge are encouraged.
Comments and tips
Dissection of the pulmonary artery
Certain techniques can be used to avoid injuries of the pulmonary artery, including handling the adventitia instead of grasping the vessel directly, and using blunt dissection instead of placing a right angle clamp behind the vessel until it has been circumferentially dissected. Some surgeons emphasize that the safest instrument to use to encircle the pulmonary artery is their own index finger (Figure 4).
The vessels are then occluded and divided with a linear cutting vascular stapler. Alternatively, in cases where the placement of the linear stapler is not feasible, an anvil non-cutting stapler is sometimes easier to pass under the vessel. The anvil of the stapler can be directed across the vessels with the guidance of a right angle clamp advanced from the opposite end. Wide dissection of the vessel is needed to allow two separate fires of the stapler and the ability to cut in between them (Figure 5). Another option is clamping and oversewing the medial portion of the vessel with a Blalock stitch using a permanent monofilament suture (Figure 6).
It is wise to assess whether the patient can tolerate loss of the lung by test clamping the pulmonary artery for a minute or two and ensuring there are no signs of the development of right heart failure. The anesthesiology team should be warned to watch for development of sinus tachycardia, supraventricular arrhythmias and signs of bilateral ventricular strain in the electrocardiogram. In such instances, pharmacological support of the right ventricle and reduction of pulmonary vascular resistance without lowering the systemic blood pressure are advised.
Intrapericardial pneumonectomy
If the patient has (I) extensive fibrosis and inflammation after radiation, (II) a tumor encroaching on any of the main vessels close to the pericardial reflection or (III) pericardial involvement by bulky tumors, proximal vascular control can be obtained by intrapericardial dissection. The pericardium should be opened longitudinally from below the inferior pulmonary vein to above the pulmonary artery posterior to the phrenic nerve to spare it. The vessels can then be easily isolated and encircled.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.08). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
Cite this article as: Avella Patino D, Ferguson MK. Right pneumonectomy. Shanghai Chest 2017;1:10.


