Right side lobectomies
Introduction
Lobectomy remains the “how to do it” type of resection because assures removal of the regional lymph nodes and thus provides the best information for staging and local control. Doing less than a lobectomy must be considered a compromise because a wedge excision does not include the lobar bronchus, impeding evaluation of lobar lymph nodes, but also usually it provides only a minimal parenchymal margin and, thus, is accompanied by a significant incidence of local recurrence. Before the operation, at a minimum, patients should have a recent chest CT scan. Most, if not all, should have a Positron Emission Tomography (PET) scan. An important aspect of the preoperative assessment of a patient with lung cancer is the assessment of pulmonary function. Preoperative spirometry to measure flows and volumes should be performed including forced expiratory volume in 1 second (FEV1), diffusive capacity, FEV1/forced vital capacity (FVC) ratio, and the ratio of the residual volume (RV) to total lung capacity (TLC). FEV1 <40% of predicted has been associated with increased postoperative morbidity and mortality. A reduced diffusing capacity has also been related to postoperative morbidity. In addition to pulmonary function studies, a valuation of exercise capability for the patient with compromised lung function may be appropriate in some circumstances. This evaluation may range from something as simple as having the patient climb one or two flights of stairs while monitoring oxygen saturation and pulse rate to, better, formal exercise testing and calculation of maximal oxygen consumption (VO2 max). Nevertheless, we can be reasonably sure that a patient who can walk up two flights of stairs can tolerate a lobectomy. For indeed borderline patients, measurement of VO2 max may be the deciding test. A value of <15 ml/kg/min has been associated with significantly increased postoperative morbidity and mortality. Patients in this category should be scrutinised carefully before one decides to proceed with resection. Other parameters suggesting high risk included a pCO2 >45 mm Hg and elevated PA pressures (1,2).
Operative techniques
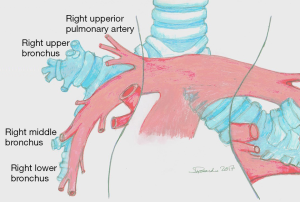
Lobectomies are approached via standard lateral decubitus position. The pleural cavity is entered through the fifth intercostal space or the bed of the fifth rib using a muscle sparing thoracotomy if possible. If adhesive pleuritis are anticipated, entrance through the bed of the resected fifth rib allows for more expeditious mobilisation of the lung, either in the intrapleural or extrapleural plane. Finger dissection manages weblike avascular adhesions and a sponge stick; cautery should be applied for vascular adhesions. Lesions adherent to the parietal pleura is mobilised in the extrapleural plane. The key to an orderly lobectomy is an accurate knowledge of the anatomy of the PA, the variations of branching, and its proper dissection (Figure 1) (2).
Right upper lobectomy
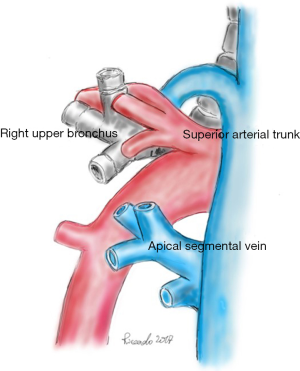
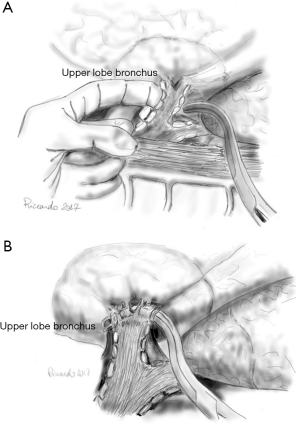
The anatomy of the hilar structures of the right upper lobe is more involved than in any other lobe, and, fortunately, arterial anomalies are more common. Usually, the anterior segment of the right upper lobe is partially or wholly fused to the middle lobe. The mediastinal pleura is sectioned around the hilum, lateral to the superior vena cava, inferior to the azygos vein, going posteriorly over the bronchus, anterior to the vagus nerve (visible subpleural), to the level of the bronchus intermedius. Anteriorly, the incision is carried to the degree of the superior pulmonary vein (PV), posterior to the phrenic nerve. The azygos vein superiorly is pushed with a pledget dissector, demonstrating the upper border of the right main bronchus and the top lobe bronchus originating from it. Inferior to the azygos-cava junction, a lymph node is found and below this the upper border of the PA. The tissue overlying the PA is dissected, and the superior arterial trunk is visualised. This artery and apical and anterior segmental branches are dissected; the apical segmental vein crosses the anterior segmental artery, and it should be ligated and divided before dealing with the artery. The superior arterial trunk is doubly tied or separated by a stapling device; the apical and segmental branches are linked and then divided (Figure 2). After the division of the superior trunk of the PA, the common stem of the apical anterior segmental veins is dissected and divided. The interlobar trunk of the PA lies directly underneath the upper and middle stems of the superior PV, and this dissection must be cautious. The remaining arterial supply to the right upper lobe is the posterior ascending artery and dissection can be the most difficult task in the procedure. The anterior approach requires the prior division of the posterior and inferior venous tributaries of the superior vein, strictly applied to the anterior surface of the inferior trunk of the Pulmonary Artery (PA). Isolation of the right PA may be required because of laceration of the posterior ascending artery or the interlobar artery during this dissection. An approach to the posterior segmental artery on the oblique fissure is acceptable if the oblique fissure is virtually complete (Figure 3). Otherwise, PA branch is again at risk for injury. The retrograde method for completion of the dissection is both harmless and quick. Retrograde exposure of the posterior ascending artery proceeds with the grasping and retraction of the vagus nerve, demonstrating arterial branches to the right upper lobe who are divided. Deep to the vagal branches, the bronchial artery may be observed (clipped and divided). The lower border of the upper lobe bronchus is then dissected. In the cross between the upper lobe bronchus and the intermediate bronchus is a constant lymph node; his dissection toward the specimen, clear the inferior border of the right upper lobe bronchus. It is not safe to pass a clamp from the lower edge of the right upper lobe bronchus medially to encircle the bronchus for the risk of laceration of the posterior ascending artery. Scissor dissection of the bronchus medial surface is performed. The bronchus is not uncovered of its fascia, and a finger can be inserted along the anterior aspect to reach its lower border (Figure 4). The bronchus is stapled and divided or manually sutured. The cut edge of the specimen side of the bronchus is grasped and elevated to facilitate the fissure dissection. With medial traction on the bronchus, the areolar tissue and nodes are dissected off the interlobar PA, and the posterior ascending artery is identified, ligated, and divided. The fissures should be managed by sharp dissection along the intersegmental vein using partial inflation of the middle and lower lobes against the upper lobe, by stapled division, or by a combination of both methods. With the bronchus divided and the posterior segmental artery transected, it is safe to pass a stapling device to separate the posterior aspect of the oblique fissure. The minor fissure is similarly completed. Medial traction of the bronchus clamp and dissection of the interlobar artery under direct vision lead to the middle trunk of the superior pulmonary vein and its posterior and inferior branches. The common stem of the posterior and inferior veins is identified. The site of insertion of the middle lobe vein into the superior PV is preserved. The venous stem is divided with a stapling device. After the specimen is removed, the pleural cavity is irrigated, and the bronchial closure is tested. The inferior pulmonary ligament is divided, allowing rotation of the lower lobe to facilitate the complete fill of the pleural space (2-5).
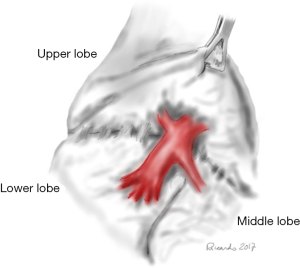
Right middle lobectomy
The inferior pulmonary ligament is mobilised. The right middle lobe lies against the anterior chest wall. The mediastinal pleura is incised to identify and ligate the right middle lobe vein, usually a branch of the right superior PV. The fissure between the upper and middle lobe is typically incomplete. After the exposition of anterior mediastinum and anterior surface of the upper and middle lobes, the venous drainage of these two lobes is recognised. The fissure line is developed as described previously. The development of the fissure between the upper and lower lobes reveals the lymph nodes in the area and the mean pulmonary artery. The right middle lobe PA arises from the intermediate artery near the superior segmental artery of the lower lobe. The right middle lobe PA is defined and stapled. The surgeon should avoid the posterior recurrent ascending branch to the upper lobe or the superior segmental branch to the lower lobe. All these vessels come off very close to one another. Finally, the right middle lobe bronchus is closed with a stapling device. Care must be taken to avoid injury to the superior segmental bronchus which comes out very close to the right middle lobe orifice. After the specimen is removed, the pleural cavity is irrigated, and the bronchial closure is tested (2,3,6).
Right lower lobectomy
The oblique fissure is opened retracting the right upper and middle lobes anteriorly and the lower lobe posteriorly. The interlobar PA is deeply situated in the region where the oblique and horizontal fissures meet. The visceral pleura overlying the interlobar artery is opened. The PA is dissected. The middle lobe artery, originating from the anteromedial surface of the interlobar artery, must be demonstrated. The superior segmental artery lies posterolaterally. Rarely, the posterior ascending artery to the upper lobe arises from the superior segmental artery. Occasionally, the basal arteries may have a short common trunk from which two branches arise: one for the anterior and medial segments, the other the posterior and lateral segments. Attention is then directed to securing the superior segmental artery, taking care to preserve the posterior segmental artery to the right upper lobe. The lobe is retracted anteriorly and superiorly. The inferior pulmonary ligament is divided up to the lower border of the inferior PV. The posterior mediastinal pleura is incised over the posterior surface of the inferior PV, which is cleared of tissue, and the pleural incision is carried superiorly to the bronchus intermedius. The interval between the lower border of the bronchus and the superior PV is dissected. The anterior surface of the inferior PV is then cleared. With a finger serving as a guide, the inferior PV is then isolated and closed by a vascular stapler. The lower lobe bronchus is then dissected. Since the middle lobe bronchus and the superior segmental bronchus derive from the intermediate bronchus, it may be necessary to divide the basal segmental bronchus and the superior segmental bronchus separately to avoid the obstruction of the middle lobe bronchus. Usually, an oblique application of the stapling device does not occlude the middle lobe bronchus. On the contrary, the lower lobe bronchus may be sutured as previously described. After the specimen is removed, the pleural cavity is irrigated, and the bronchial closure is tested (1,3,4).
Bilobectomy
Sporadically, the location of a lesion dictates removal of the middle and lower portions, a procedure that can be accomplished en bloc because of the common origin of these lobes as the bronchus intermedius. Where an indication exists for bilobectomy the vessels for each lobe are isolated and divided as described for each lobectomy. Once the PA branches have been divided the point of separation of the bronchus becomes apparent; the bronchus should be divided above the origin of the middle lobe bronchus just distal to the upper lobe take-off. If upper and intermediate lobes are resected, the bronchi are separately closed. For middle and lower lobectomy, the bronchus intermedius is divided distal to the right upper lobe bronchus. The major fissure is opened, and the lower lobe is retracted posteriorly. By following the posterior edge of the middle lobe as it joins the major fissure, and dissecting within the fissure, lymph nodes are evidentiated, indicating the site of the interlobar PA. The artery is dissected in the subadventitial plane, and the middle lobe artery is identified. Two middle lobe arteries exist: one defined arises from the interlobar artery anteriorly, opposite to the superior segmental artery. Proximal dissection of the interlobar artery shows a second (rarely a third) artery to the middle lobe. After the division of the middle lobe vein, the bronchus is readily accessible. After the sample is removed, the pleural cavity is irrigated, and the bronchial closure is tested (3-5).
Comments
There are several significant anatomic features relating to right-sided pulmonary resections that are unique to the right chest. The right main PA is relatively long and courses posterior to the superior vena cava and across the carina. This extra length of the artery is an advantage for some proximal lesions that in a comparable location on the left side would not be resectable because of the short length of the left main PA about the bifurcation. There should be enough length of artery distal to the bifurcation to be able to place a clamp and divide and suture the artery so that the lesion can be removed. The distance between the carina and the origin of the right upper lobe bronchus usually is <2 cm, and the Carina is readily mobilised from the right side. Access to the proximal left mainstem bronchus is significantly easier from the right side compared to the left side. The superior mediastinum is well visualised from the right side. The area bounded by the azygous vein (inferior), the trachea (posterior), the subclavian vein (superior), and the superior vena cave (anterior) delineates this compartment, whose lymph node bearing contents may be removed en bloc from the right side. In evaluating resections from the right side, the upper lobectomy probably is the most straightforward resection, although the location of the posterior segmental arterial branch may, at times, be problematic. Right lower lobectomy is complicated by the site of the middle lobe artery and bronchus. Major nonfatal events most commonly are respiratory related with patients developing significant infiltrates and pneumonitis. A small percentage of patients require reintubation in the postoperative period for respiratory failure usually related to the development of an infiltrate. Pulmonary complications can be minimised with meticulous attention to postoperative respiratory manoeuvres including chest physiotherapy and preoperative teaching. Cardiac complications also account for a significant percentage of mortality, whereas technical problems such as haemorrhage, bronchopleural fistula, and empyema account for a small but important percentage of complications leading to death. The most common minor complication is a supraventricular arrhythmia depending on how closely patients are monitored. Most of these respond to simple pharmacologic manipulation and rarely are hemodynamically significant at onset. With appropriate treatment, the rhythm reverts to sinus rhythm quickly, and patients may be taken off the antiarrhythmic drugs usually after one month. Other minor complications include postoperative air leaks lasting more than seven days and atelectasis. Meticulous attention to detail in all phases of management preoperative, intraoperative, and postoperative goes a long way toward keeping problems to a minimum (1-3).
Acknowledgments
The authors would like to thank Dr Eng. Riccardo Pardolesi for the indispensable help in the elaboration of the figures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci, Alan D.L. Sihoe and Benedetta Bedetti) for the series “Open Thoracic Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2017.05.09). The series “Open Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumgartner F. Cardiothoracic Surgery (Vademecum), 3rd Edition. CRC Press, 2004.
- Shields TW, Lo Cicero J, Ponn RB, et al. General Thoracic Surgery, 6th Edition. Lippincott Williams & Wilkins, 2005.
- Kaiser LR, Kron IL, Spray TL. Mastery of Cardiothoracic Surgery, 2nd Edition. Lippincott Williams & Wilkins, 2007.
- Riquet M, Le Pimpec Barthes F. Linfectomie nel corso delle exeresi polmonari per cancro. EMC – Tecniche Chirurgiche Torace 2012;16:1-12.
- Renaud S, Renaud C, Seguin A, et al. Principi della chirurgia di exeresi polmonare. EMC – Tecniche Chirurgiche Torace 2013;17:1-9.
- Ellison EC. Zollinger’s atlas of surgical operations, Tenth edition. McGraw – Hill, 2016.
Cite this article as: Bertolaccini L, Pardolesi A, Solli P. Right side lobectomies. Shanghai Chest 2017;1:7.