Robotic thymectomy: current perspective in myasthenia gravis and thymoma
IntroductionOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
Thymectomy is the most frequent surgical procedures involving the anterior mediastinum. Although it is an established operation both for benign and malignant diseases of the thymus, many issues are still ongoing or controversial, both in terms of procedural indications and of surgical technique.
Removal of the thymic gland is nowadays a well-established surgical approach in the management of myasthenia gravis (MG) and, at the same time, thymectomy represents the main treatment for thymic tumors, particularly in early stage disease (1,2).
From the surgical point of view, different surgical approaches have been described for thymectomy with median sternotomy still being considered the gold standard. However, the complications and the mortality related to this technique led to the development of minimally invasive approaches in this surgical field, especially for benign diseases.
Robotic surgery has been developed as an evolution of standard video-assisted thoracoscopic surgery (VATS). Advantages of robotic technique over other minimally invasive approaches become clear particularly for procedures in a surgically challenging anatomical region, such as the anterior mediastinum (3).
After the first surgical applications of the robotic technique mainly in the cardiac surgery field, in 2001 Yoshino described the first robotic thymectomy for a thymoma (4-6). Since this first experience, various studies have been published describing the application authors of robotic thymectomy both for nonthymomatous MG and for thymic tumors (1,3,7-27).
MG and robotic thymectomyOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
MG is an autoantibodies-mediated disease involving the neuromuscular junction, characterized by weakness and fatigability of skeletal muscles. The incidence of MG is 2.5–10 per 1,000,000 and it’s more common in women between 20 and 40 years old and in people of both sex between 50 and 60 years old, showing a double peak prevalence. Moreover, MG is the most frequent paraneoplastic syndrome associated to thymomas, in about 30–40% of cases (28).
Approximately in 80–90% of the patients affected it is possible to find anti-acetylcholine receptor (AChR) antibodies. The link between AChR antibody and AChR results in an alteration of the neuromuscular transmission, which is responsible of the clinical picture (29). About 10–15% of MG patients are sero-negative for AChR antibodies: in this subgroup of patients it is possible to detect, in 40–70% of the cases, another type of antibody direct against the muscle-specific tyrosine kinase (MuSK) (30-33).
The treatment of MG is composed by several options that include pharmacological therapy, plasmapheresis and surgery. Within the pharmacological therapy, cholinesterase inhibitors and immunomodulating drugs represent the mainstay of treatment. Among the former the most used is pyridostigmine (Mestinon), which is frequently associated with corticosteroids. Intravenous immunoglobulin or plasmapheresis are used in order to treat myasthenic crisis or severe exacerbations of the disease (34).
Thymectomy represents the surgical therapeutic option. Since the first experiences described by Blalock, thymectomy has been considered part of the multidisciplinary treatment of MG, although only recently a prospective randomized clinical trial demonstrated the benefits of thymectomy in myasthenic patients (35). Currently, thymectomy is indicated in all myasthenic patients with thymoma or patients affected by non-thymomatous generalized MG. In case of non-thymomatous ocular MG, the surgical approach is still source of debates (9,36,37). Thymectomy should be performed in patients with stable disease, particularly with no recent crisis, and shows increased benefits when performed early after the onset of the symptoms (38). There is also evidence that thymectomy may have less effects in patients with presence of anti-MuSK antibodies (39).
The aim of surgery in myasthenic patients is the complete removal of the thymus, together with the surrounding mediastinal fat, frequently containing foci to ectopic thymus, since the completeness of resection of as much as possible of thymic tissue is associated with higher improvement rate (40).
First thymectomies were performed through median sternotomy. Anyway, the increased risk of respiratory failure, myasthenic crisis and the concerns regarding the sternal healing impairment in myasthenic patients with chronic steroids use, led to the introduction of minimally invasive approach, such as cervicotomy and VATS thymectomy (41).
The first descriptions of robotic thymectomy for myasthenia were performed in 2003 by Ashton et al. and Rea et al. (42,43). Since these first experiences, different authors reported their experience with robotic approach for myasthenic patients showing results comparable with open approaches, both in terms of surgical and neurological outcomes, with an improvement rate of symptoms ranging between 77% and 90% and complete remission rate between 16.2% and more than 40% (Table 1).
Table 1
| Author | Number of patients | Thymoma/non-thymoma (n) | MG (n) | OC (%) | OT (min) | Morbidity (%) | POS (days) | FU (months) | RR (%) | Thymoma 5-year survival (%) | MG remission (CRS, PR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rea et al., 2006 (1) | 33 | 2/31 | 33 | 0 | – | 6.0 | 2.6** | 28.8* | 0 | – | 16.6% CSR |
| Cakar et al., 2007 (7) | 9 | 4/5 | 9 | 0 | 154.0* | 11.1 | 5.0* | 13.0** | – | – | 100% I |
| Augustin et al., 2008 (8) | 32 | 6/26 | NA | 3.1 | – | 6.2 | 6.0** | 25.0* | 0 | – | – |
| Ruckert et al., 2008 (9) | 95 | 12/83 | 95 | 8.3 | 186.0* | 2.1 | – | 29.1** | 0 | – | >40% CSR |
| Fleck et al., 2009 (10) | 18 | 0/18 | 18 | 5.5 | 175.0* | 33.3 | 4.2* | 18.0** | – | – | 55.5% |
| Goldstein et al., 2010 (11) | 26 | 5/21 | 26 | 15.3 | 127.0* | 15.3 | 2.0* | 26.0* | – | – | 38.0% PR |
| Balduyck et al., 2011 (3) | 14 | 5/9 | 5 | 7.1 | 224.2* | 21.4 | 9.6* | 34.2* | – | – | – |
| Freeman et al., 2011 (12) | 75 | 0/75 | 75 | 0 | 113.0* | 6.6 | 2.2* | 45.0* | – | – | 53.3% |
| Ruckert et al., 2011 (13) | 74 | 11/63 | 74 | 1.4 | 198.0* | 2.7 | – | 42.0* | – | – | 39.3% CSR |
| Mussi et al., 2012 (14) | 14 | 14/0 | 1 | 7.7 | 139.0* | 14.2 | 4.0* | 14.5** | 0 | 100 | 100% I |
| Melfi et al., 2012 (15) | 39 | 13/26 | 20 | 5.1 | 124.3* | 12.8 | 4.3* | 16.0** | 0 | – | 90.0% I |
| Marulli et al., 2012 (16) | 79 | 79/0 | 45 | 1.3 | 165.0* | 12.7 | 4.4* | 51.7* | 1.3 | 97 | – |
| Weksler et al., 2012 (17) | 15 | 10/5 | 5 | 0 | 84.0* | 6.6 | 1.0** | – | – | – | – |
| Marulli et al., 2013 (18) | 100 | 8/92 | 100 | 0 | 120.0** | 6.0 | 3.0** | 67.0** | 0 | 100 | 28.5% CSR; 87.5% I |
| Schneiter et al., 2013 (19) | 20 | 20/0 | 12 | 0 | – | 10.0 | 5.0** | 26.0** | 11.1 | 100 | – |
| Ye et al., 2014 (20) | 23 | 23/0 | 0 | 0 | 97.0* | 4.3 | 3.7* | 16.9* | 0 | 100 | – |
| Keijzers et al., 2014 (21) | 37 | 37/0 | 28 | 13.5 | 149.0* | 16.2 | 3.0** | 36.0** | 2.7 | 100 | 21.4% |
| Seong et al., 2014 (22) | 34 | 11/23 | 2 | – | 157.2* | 0 | 2.7* | 13.3* | 0 | – | – |
| Jun et al., 2014 (23) | 55 | 21/34 | NA | 0 | 139.8* | 10.9 | 7.2* | – | – | – | – |
| Huang et al., 2014 (24) | 23 | 23/0 | 1 | 0 | 85.2* | 4.3 | 3.6* | 24.8* | 0 | – | 100% I |
| Keijzers et al., 2015 (25) | 125 | 31/94 | 125 | 4 | 125** | 7.2 | 3.0** | 33.0** | 6.5 | – | 77% I |
| Marulli et al., 2016 (26) | 134 | 134/0 | 70 | 8.9 | 140** | 17.1 | 4.0** | 48.0* | 0.7 | 100 | – |
| Kumar et al., 2017 (27) | 71 | 21/50 | 71 | 0 | 140** | 7.0 | – | 33.0** | 0 | – | 38% CSR |
*, mean; **, median. MG, myasthenia gravis; OC, open conversion; OT, operative time; POS, post-operative stay; FU, follow-up; RR, recurrence rate; CSR, complete stable remission; PR, pharmacologic remission; I, improved; NA, not available.
Thymoma and robotic thymectomyOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
Thymomas are generally considered rare tumors, representing less than 1% of all malignancies, but account for more than 50% of all anterior mediastinal neoplasms (44). Surgery is the main treatment of thymoma, particularly for Masaoka stages I and II, and completeness of resection is the most important factor for patients’ prognosis (45,46). The established and most widespread surgical approach is represented by median sternotomy, which has the advantages of allowing in most cases a technically easy and oncologically safe operation (47).
As in other surgical fields, in recent years, minimally invasive techniques (in particular the thoracoscopic approach) have gained popularity in the surgical treatment of neoplastic diseases of the mediastinum, for their capability to assure a complete removal of the tumor, without the disadvantages of the open technique. Anyway, several surgeons have been hesitant to use thoracoscopic approach for thymic tumor resection, because it is considered a difficult operation requiring a long learning curve. Other additional issues that slowed the diffusion of VATS resection for thymomas are represented by the possible rupture of the capsule during endoscopic maneuvers, the supposed reduced safety margins with increased risk of recurrence and the lack of long-term oncologic results (26).
Different authors have published their experience with the robotic approach for thymic tumors (Table 1). Analysis of current literature data shows that robotic thymectomy may be considered a technically sound and safe operation in the hands of trained surgeons: there are no major complications reported and no mortality occurred, whereas the mean rate of open conversion is 6.6%.
Recently, Friedant et al. addressed this issue performing a systematic review of the available literature regarding VATs and robotic thymectomy versus open approach for Masaoka stage I and II thymic tumors. Authors concluded that, based on current data, there are no significant differences in terms of R0 resection and locoregional recurrence between the two groups (48).
These data are promising regarding the robotic/thoracoscopic treatment of Masaoka stage I and II thymomas whereas for advances stages, although technically feasible, no clear data or indications are available, at the moment. Therefore, extended resections should still be considered experimental and reserved to very selected cases (26).
Focusing on the oncologic outcome, these reports show a low recurrence rate (0–11.1%), comparable with open approaches. However, these series have several limitations based on their retrospective fashion, a relatively small sample size and a short oncologic follow-up. These issues make current data promising, but further studies with longer follow up are needed, particularly for tumors such as thymomas that show a relatively indolent nature and a long recurrence time.
Robotic systemOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
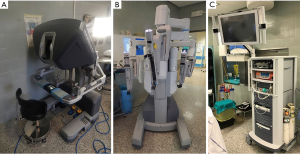
The da Vinci (Intuitive Surgical, Inc) is the most widespread robotic system nowadays.
The system includes a console where the surgeons sits while operating, a cart that supports the interactive robotic arms and a magnified vision system. The surgeon operates at the console far from the operating table. The console is connected both to the video system, giving the surgeon, through a binocular localized in the upper part of the console, a high-definition, three-dimensional (3D) view inside the patient’s body, and to the robotic cart. The system allows the surgeon’s hand movements to be translated into identical, smaller and precise movements of the instruments inside the patient’s chest (Figure 1).
Surgical techniqueOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
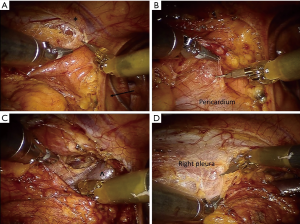
The patient is under general anesthesia and has an endotracheal double-lumen tube for selective single lung ventilation during the time of operation. The patient is positioned supine at a 30-degree angle upon a bean bag. For the left side approach, the left arm is positioned on a support parallel to the bed, to allow an easier exposure of the axillary region, whereas for the right-side approach it is the opposite. Considering an eventual emergency conversion to an open approach (sternotomy or thoracotomy), the operative field is always draped including the entire hemithorax and the sternal region. Ports are introduced as follows: first the camera port through a 15-mm incision on the 5th intercostal space in the anterior portion of the midaxillary region. Then, aided by direct internal vision, the other two ports are inserted, one on the 3rd intercostal space on anterior portion of the midaxillary region and another on the 5th intercostal space on the midclavicular/parasternal line (Figure 2). Subsequently, the arms of the da Vinci system are attached to the ports, the middle one to the port-inserted endoscope and the other two to the remaining access points. The right arm is generally connected to a dissector device (electric cautery or an ultrasound dissector) while the arm has an atraumatic grasper. Through the camera port, the hemithorax is inflated with CO2 (pressure range 6–10 mmHg) to allow an easier dissection in the mediastinal region. Before beginning the dissection, the pleura is carefully explored to exclude any unexpected pleural lesion in case of thymic tumor. When a left-sided approach is performed, the dissection starts from the fat tissue at the left cardiophrenic angle and moves upwards along the anterior border of the phrenic nerve, isolating all mediastinal tissue from the nerve. Subsequently, the thymic gland is separated from the retrosternal area (Figure 3A). The left inferior horn of the thymus is then located and dissected from the pericardium (Figure 3B), allowing the lower part of the gland to be mobilized upwards and the thymic tissue is dissected from the aorto-pulmonary window. The dissection continues on the right side with the visualization of right mediastinal pleura and the right inferior horn is found and dissected. The left innominate vein is then identified, and the dissection proceeds along the antero-superior border of the vein from the left to the right side. Keynes veins are identified, clipped, and divided. Concurrently, dissection continues upward to the neck until the left superior horn and then the right one are also identified and divided from the thyroid gland thus completing the dissection (Figure 3C,D). The resected specimen is placed in an Endobag, so that it can be entirely removed through the parasternal trocar incision. In case of predominantly right side thymoma, a right-side approach may be considered. In this case, as for the left side, the dissection of the thymus begins inferiorly from the cardiophrenic angle and moves upwards anteriorly following right phrenic nerve, separating all anterior mediastinal tissue from the nerve. Then, the pleura is divided all along the mammary vessels, from their origin to the diaphragm, thus dissecting the mediastinal tissue from the sternum. In the next step, the inferior horns of the thymus are dissected from the pericardium and then all the gland is elevated form the pericardium until the left innominate vein is encountered. The dissection moves along the anterior-superior border of the vein and the Keynes veins are identified, clipped, and divided. Concurrently, dissection continues upward to the neck until the left superior horns are also identified and divided from the thyroid gland. The most delicate step is the dissection of the thymus on the left side, identifying the left phrenic nerve by opening the left pleura. After hemostasis, a 28-Fr drain tube is placed through the port in the fifth intercostal space. Generally, 24 hours after operation it is possible to remove the chest drain if the postoperative chest X-ray shows normal findings and the amount of pleural fluid is permissive.
Recently, Suda and colleagues described a novel procedure by combining the subxyphoid approach performed through a midline camera port with the use of the robotic system. According to the authors, this approach would provide a good operative view in the neck region and enables an easy verification of both phrenic nerves (49).
Anyway, the main debate is whether it is better to perform thymectomy from the right or the left side. Surgeons supporting the left-sided approach point out that usually the left lobe of the thymus gland is larger than the right one and that the most frequent sites of ectopic thymic tissue may be found the aortopulmonary window and below the left brachiocephalic vein are frequent sites of ectopic thymic tissue. In some cases, the thymus may show some anatomical variations, spreading lateral to or under the left phrenic nerve, or descending posteriorly to the left brachiocephalic vein. Moreover, when dissecting the thymus from the left side, the right phrenic nerve is hardly damaged, being protected by the superior vena cava (13,50).
On the contrary, surgeons supporting the right-side approach describe the advantages of a larger operative field and a safer entry of the instruments because of the absence of the heart, allows the improved visualization of the aortocaval groove and of the venous confluence. However, the left phrenic nerve cannot be always completely visualized. Anyway, according to these authors, this approach is safer especially at the beginning of the learning curve (50-52).
Another issue regards the extent of surgical resection, particularly thymomectomy versus thymo-thymomectomy. According to the recent International Thymic Malignancy Interest Group (ITMIG) guidelines, thymomas should be removed together with the surrounding thymus and fatty tissue, particularly in case of myasthenic patients, to gain safety margins and reduce the risk of incomplete resection and local recurrence also for apparently capsulated thymomas (53). Indeed, clinical stage I thymomas are frequently found to be upstaged at final pathological examination, with subtle microscopically or macroscopically invasion of the surrounding tissue that may not be detected by the surgeon (53,54).
Moreover, surgeons should resect the thymoma using a “no-touch” technique, avoiding to grasp or squeeze the tumor with the endoscopic instruments because of the increased risk of capsule’s rupture and of consequent pleural dissemination of tumor cells (53). This “no-touch” technique requires obviously a more precise and complicated dissection, thus longer operative time and learning curve for the surgeon.
To better select candidates, Cheng and colleagues suggested some ideal radiological criteria supporting a robotic/VATS approach for resection of thymic tumors: the evidence of a tumor capsule, a distinct fat plane separating the tumor from the thoracic organs with no mass compression effect, the presence of residual normal thymic tissue surrounding the lesion, and the unilateral tumor predominance, particularly for lesions greater than 3 cm (Figure 4) (55).
Anyway, the right dimension of thymic tumors for robotic/thoracoscopic approach, it is still matter of debate, but generally an upper cut-off of 5 cm is considered acceptable (16,56). A large dimension of the thymoma is not an absolute contraindication, however, it could make the endoscopic manipulation more difficult, increase the possibility of an open conversion or prolonged the operative time (16). This eventuality should be considered also in case of myasthenic patients with a small lesion but rather abundant mediastinal fat.
When compared to other surgical approaches, robotic thymectomy shows particular advantages compared to both sternotomy and standard VATS.
In literature, the comparison between robotic and transsternal thymectomy has been made only by few authors (3,7,17,20,22). The first series comparing the two approaches was made by Cakar and colleagues and showed a better clinical outcome, particularly a lower complications’ rate and a reduction in the post-operative stay, in patients who underwent robotic surgery (7). Balduyck and colleagues also compared the two techniques focusing on quality of life assessment, by means of specific questionnaires, and showed a better post-operative recovery in the robotic group (3). Seong et al. and, more recently, Kang et al. compared sternotomy and robotic thymectomy using a propensity score matching (22,57). Both studies showed a superiority of robotic approach over sternotomy in terms of surgical outcome and promising results in the intermediate follow up from the oncological point of view. It is clear that median sternotomy, although assuring a complete and safe removal of the thymic gland, shows concurrently some specific complications, as wound healing impairment and infections, sternal instability, hematomas, that are avoided or very rare with the robotic approach. The lower morbidity associated with the robotic approach has led to a greater acceptation of this operation, particularly in myasthenic patients, where the use of steroids and the respiratory impairment related to myasthenia, make higher the risk of complications.
In the field of minimally invasive techniques, the robotic approach is considered as a development of VATS. Anyway, to date only few studies were focused on a real comparison between these techniques. Published results showed similar surgical results, except for the operative, time hospitalization costs (increased for robotic approach) and length of hospital stay and (longer for VATS approach) (13,23,58,59). Only Rückert et al. showed also a better outcome for myasthenic patients after robotic thymectomy (13). Qian and colleagues recently performed a comparison between VATS, robotic approach and sternotomy in the treatment of early stage thymomas. This study showed better post-operative outcome, particularly in terms of duration of hospital stay and post-operative pleural drainage volume, in patients treated through the robotic approach compared to median sternotomy and also to VATS group (59).
Anyway, no prospective randomized trials comparing outcomes based on different surgical approach are available and it is unlikely that they will be performed in the future. Therefore, preference between one or the other technique is based mainly on impressions and personal feeling than on scientific data. It is doubtless that the robotic approach, while displaying all the advantages of minimally invasive approaches, concurrently seems to overcome the disadvantages of standard VATS as the bidimensional vision, the less than ideal range of motion of the long thoracoscopic instrument placed through fixed entry points, thus creating a fulcrum effect, and the transfer of the surgeon’s tremor. On the contrary, the 3D view, the articulated instruments with 360-degrees of rotation, the 7 degrees of freedom and the tremor filtering system seem to minimize difficulties in cases of challenging thymectomies (60). Apart from technical advantages, the robotic approach seems to have a shorter learning curve than that of standard VATS thymectomy, that, on the contrary, has always been considered a technically demanding operation (13,15,23,56,60).
Some financial concerns have been raised regarding the robotic surgery: as evaluated by Augustin and colleagues, conventional thoracoscopy is significantly cheaper compared to the robotic approach, with additional cost of about 91% for the robotic technique, mainly related to the initial capital costs of the robotic system, the need of annual maintenance and the disposable surgical materials (8). High cost may be partially compensated by the multidisciplinary use of the system, by fewer complications rates and a reduced post-operative stay, but to date no specific analysis on this issue has jet been performed (17).
Other disadvantages seem the lack of tactile feedback, that anyway seems to be widely compensated by the superior 3D view of the operating field, and the placement of the surgeon, away from the patient and operating at a non-sterile console (9,17).
ConclusionsOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
Nowadays, the robotic approach for thymectomy may be considered a safe and well-established surgical technique. Results in patients with MG are encouraging and comparable with other techniques, either open or minimally invasive ones. For patient with thymic tumors, the role of robotic thymectomy is still under evaluation but available data confirm the promising results in terms of oncologic outcomes.
AcknowledgmentsOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Domenico Galetta) for the series “Minimally Invasive Thoracic Oncological Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2018.04.08). The series “Minimally Invasive Thoracic Oncological Surgery” was commissioned by the editorial office without any funding or sponsorship. GM serves as an unpaid editorial board member of Shanghai Chest from Dec 2017 to Nov 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the noncommercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- MG and robotic thymectomy
- Thymoma and robotic thymectomy
- Robotic system
- Surgical technique
- Conclusions
- Acknowledgments
- Footnote
- References
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- Reichenspurner H, Damiano RJ, Mack M, et al. Use of the voice-controlled and computer-assisted surgical system ZEUS for endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 1999;118:11-6. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4. [Crossref] [PubMed]
- Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-71. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Fleck T, Fleck M, Müller M, et al. Extended videoscopic robotic thymectomy with the da Vinci telemanipulator for the treatment of myasthenia gravis: the Vienna experience. Interact Cardiovasc Thorac Surg 2009;9:784-7. [Crossref] [PubMed]
- Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg 2010;89:1080-5; discussion 1085-6. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Van Woerkom JM, et al. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. Ann Thorac Surg 2011;92:1018-22; discussion 1022-3. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6. [Crossref] [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Schneiter D, Tomaszek S, Kestenholz P, et al. Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur J Cardiothorac Surg 2013;43:288-92. [Crossref] [PubMed]
- Ye B, Li W, Ge XX, et al. Surgical treatment of early-stage thymomas: robot-assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc 2014;28:122-6. [Crossref] [PubMed]
- Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years' experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Jun Y, Hao L, Demin L, et al. Da Vinci robot-assisted system for thymectomy: experience of 55 patients in China. Int J Med Robot 2014;10:294-9. [Crossref] [PubMed]
- Huang P, Ye B, Yang Y, et al. Experience with the "da Vinci" robotic system for early-stage thymomas: Report of 23 cases. Thorac Cancer 2014;5:325-9. [Crossref] [PubMed]
- Keijzers M, de Baets M, Hochstenbag M, et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur J Cardiothorac Surg 2015;48:40-5. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Kumar A, Goyal V, Asaf BB, et al. Robotic thymectomy for myasthenia gravis with or without thymoma-surgical and neurological outcomes. Neurol India 2017;65:58-63. [PubMed]
- Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2010;37:13-25. [Crossref] [PubMed]
- Capone L, Gentile R, Schoenhuber R. Thymus and myasthenia gravis. Pathophysiological and clinical features. In: Lavini C, Moran CA, Morandi U, et al. editors. Thymus gland pathology. Springer, Milano, 2008:89-98.
- Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365-8. [Crossref] [PubMed]
- Liyanage Y, Hoch W, Beeson D, et al. The agrin/muscle-specific kinase pathway: new targets for autoimmune and genetic disorders at the neuromuscular junction. Muscle Nerve 2002;25:4-16. [Crossref] [PubMed]
- McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol 2004;55:580-4. [Crossref] [PubMed]
- Ghazanfari N, Fernandez KJ, Murata Y, et al. Muscle specific kinase: Organiser of synaptic membrane domains. Int J Biochem Cell Biol 2011;43:295-8. [Crossref] [PubMed]
- Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010;17:893-902. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. Erratum in: Randomized Trial of Thymectomy in Myasthenia Gravis [N Engl J Med 2017]. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:1319-24. [Crossref] [PubMed]
- Wong SH, Huda S, Vincent A, et al. Ocular myasthenia gravis: Controversies and updates. Curr Neurol Neurosci Rep 2014;14:421. [Crossref] [PubMed]
- Melzer N, Ruck T, Fuhr P, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol 2016;263:1473-94. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [Crossref] [PubMed]
- Zieliński M, Kuzdzal J, Szlubowski A, et al. Comparison of late results of basic transsternal and extended transsternalthymectomies in the treatment of myasthenia gravis. Ann Thorac Surg 2004;78:253-8. [Crossref] [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [Crossref] [PubMed]
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Rea F, Bortolotti L, Girardi R, et al. Thoracoscopic thymectomy with the ‘da Vinci’ surgical system in patient with myasthenia gravis. Interact Cardiovasc Thorac Surg 2003;2:70-2. [Crossref] [PubMed]
- Srirajaskanthan R, Toubanakis C, Dusmet M, et al. A review of thymic tumours. Lung Cancer 2008;60:4-13. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. [Crossref] [PubMed]
- Deen S, Farivar AS, Louie BE. Thoracic techniques: robotic thymectomy for thymoma. Indian J Surg Oncol 2013;4:132-7. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Takeo S, Tsukamoto S, Kawano D, et al. Outcome of an original video-assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg 2011;92:2000-5. [Crossref] [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [Crossref] [PubMed]
- Toker A, Erus S, Ozkan B, et al. Does a relationship exist between the number of thoracoscopic thymectomies performed and the learning curve for thoracoscopic resection of thymoma in patients with myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:152-5. [Crossref] [PubMed]
- Kang CH, Hwang Y, Lee HJ, et al. Robotic Thymectomy in Anterior Mediastinal Mass: Propensity Score Matching Study With Transsternal Thymectomy. Ann Thorac Surg 2016;102:895-901. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Ismail M, Swierzy M, Rückert JC. State of the art of robotic thymectomy. World J Surg 2013;37:2740-6. [Crossref] [PubMed]
Cite this article as: Marulli G, Comacchio GM, Rebusso A, Schiavon M, Rea F. Robotic thymectomy: current perspective in myasthenia gravis and thymoma. Shanghai Chest 2018;2:40.