
Innovations in surgical scopes—wireless steerable endoscopes and magnetic cameras
Introduction
Innovations in surgical endoscopes have been the key to progress in minimally invasive surgery. The development of video-assisted thoracic surgery (VATS) in the 1990s is no different, with the introduction of the rod lens endoscopic system that has revolutionized how surgery is performed. Instead of peering through an open incision, the endoscope allows a magnified, illuminated view of the operating site that has increasingly become more high definition with the years of technological progress. In addition, the development of smaller narrower thoracoscopes, high definition CCD cameras, fluorescence imaging, 3D vision systems and variable wide viewing angle endoscopes have further refined minimally invasive thoracic surgery, making it safer, more easily adoptable, less invasive and with additional applications. More recently, there has been growing interest in endoscopes that provide more angles and directions of vision, that minimize interference or fencing with the operating surgeon. This is partly driven by the quest for smaller and less surgical access wounds as well as the spread of uniportal VATS (1). We hereby present some endoscope ideas and concepts that may provide such solutions.
Cardioscope
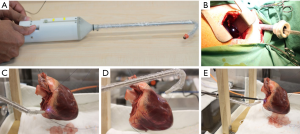
Thoracoscopes with a rigid rod lens design are commonly used in VATS to provide an illuminated view within the thoracic cavity. They offer the sharp image quality desired in endoscopic surgery, and has a beveled tip to offset the viewing angle. Surgeon can adjust the viewing direction of the thoracoscope by steering the long shaft or rotating the angled lens to gain alternative viewing angle, both of which increase the risk of colliding with other surgical instruments, and may prolong the operation time. In uniportal VATS, the problem is more severe as multiple instruments are inserted via the same crowded incision, and instrument fencing can easily occur while maneuvering the thoracoscope. Endoscopes that allow adjustment of viewing direction near the distal end can alleviate the problem by reducing rod body maneuvers (2). While the Olympus Swing Prism Borescope can achieve 120° viewing arc solely by rotation of the tip prism, it has seen more industrial application instead of surgical use, possibly due to limitations in preventions of debris contamination and sterilization (3). On the other hand, the ENDOEYE FLEX, also by Olympus, provides an articulating distal section that bends up too 100° in four directions, allowing change of viewing direction by steering the tip only, and offer 3D vision (4). However, it has large tip size, fixed bending workspace and limited bending range. To further improve field of view (FOV) of flexible endoscope and avoid collision with organs and other instruments, we developed the cardioscope (5-7) which not only has an articulated bending section, but also incorporate a rigid restriction rod that slides within the articulated section, thus changes the bendable length of the flexible instrument. The cardioscope has a smaller diameter, can achieve retrograde view behind an organ, and has an adjustable bending workspace to adapt to a varied environment and obstacles. Figure 1 shows the cardioscope prototype tested within ex vivo and in vivo set-ups. As compared to ENDOEYE FLEX, the cardioscope currently has lower image quality and does not offer 3D vision. Further research need to address these limitations, nonetheless the articulation approach with variable bending length could be applied to improve dexterity of other surgical instruments.
Soft MAGS endoscope
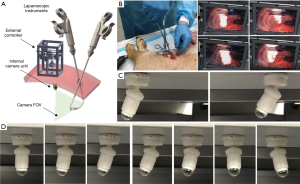
While flexible endoscopes like ENDOEYE FLEX and cardioscope can reducing fencing between endoscope and other instruments, the problem remains that the shaft body passes through a crowded incision in uniportal VATS, and limits the maneuvering of instruments (8). Replacing thoracoscope with a thin wired or wireless internalized endoscope that does not or occupies less incision space could solve the instrument fencing problem, while reducing the port size required for VATS. Magnetic anchoring and guidance system (MAGS) provides the strategy suitable for controlling a thin-wired or wireless endoscope within the thoracic cavity (9). A MAGS endoscope typically has two parts, an external magnetic component and an internal camera unit. The internal unit containing small permanent magnets is introduced via a small incision at the beginning of the surgical procedure; the unit is attracted and anchored by the external magnetic component on the intraluminal surface, and guided to slide away from the incision. The incision is therefore no longer occupied by the endoscope and could be used to insert other surgical instruments (10). This approach not only reduce the incision size and avoid fencing, but also potentially allow docking of multiple MAGS endoscopes that provide quick and convenient switching between many views of operating site. However, a major challenge in MAGS endoscope is the pan and tilt control of viewing direction. Many proposed systems either do not offer view adjustment (11-13) or utilized on-board DC motors to adjust viewing direction of internal units (14-16). These systems often suffer excessive device bulk and weight, complex assembly that increase production cost and difficulty in sterilization, and require high electrical power to drive on-board motor that increase the risk for patient. To overcome these challenges and create MAGS endoscopes more feasible for VATS, we designed a Soft MAGS endoscope that uses magnetic actuation to deform a silicone body and provide viewing direction control without on-board motors (17).
The Soft MAGS endoscope also has two components, an external controller and an internal camera unit. The external controller is a motorized box controlling the position of an external permanent magnet (EPM) in a limited workspace, whereas the internal unit is a continuous silicone body embedding two internal permanent magnets (IPMs) and a wireless capsule camera. The silicone body has three parts, a top chamber with an upper IPM contained in a cylindrical space, a lower cup contained another IPM and a capsule camera, and a middle joint connect the two. Horizontal motion of the EPM can lead the upper IPM to move in the top chamber, attracting another IPM to bend the middle joint, thus changing the viewing angle of the capsule camera. Large motion of EPM can lead the whole internal unit to slide along the intraluminal surface to a new location within the pleural cavity. Figure 2 shows the schematics of Soft MAGS endoscope, and the prototype being tested in benchtop and in vivo environment. Overall, compared to other MAGS endoscope, the system has advantages of being lightweight (16.5 g), compact (42 mm in length), simple to produce and sterilize, and safe for patient, with a soft body fabricated by silicone curing, which is able to passively adapt to delicate anatomic tissues, and does not require electrical power for any on-board motor. As for limitations, since operating space within transverse plane is highly limited in VATS compared abdominal surgery where carbon dioxide insufflation is routinely used, the workspace of Soft MAGS endoscope sweeping 42 mm vertically is not ideal. We also noted during in vivo evaluation that the low video frame-rate and battery life provided by the wireless capsule camera may not be feasible for VATS application.
Magnetic actuated endoscope
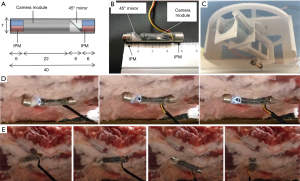
To improve upon the Soft MAGS concept while addressing its limitations, we created another magnetic actuation endoscope. Taking into account some innovations of magnetic actuation strategies using diametrically charged magnets (18), and electromagnetic coil arrays (19), we designed a magnetic endoscope aiming to improve image quality and frame rate, while limiting the workspace required by the endoscope to function.
To improve the frame rate and overcome battery limitation, a wired endoscope with on-board LED is used, where the camera is 4.5 mm in diameter, with a 20 mm circuit board. To minimize the effect of tether on endoscope actuation, and reduce the occupancy of surgical port, a thin wire bundle of less than 2 mm diameter is used for both power supply and signal transmission. The camera module is placed into a cylindrical capsule (7 mm diameter, 40 mm length), along with a 45° mirror, and two 6×6 mm2 diametrically magnetized IPMs on either ends of the capsule, forming the internal unit. The magnetic polarity of the two IPMs are aligned in parallel, such that the internal unit is anchored with its length along the intraluminal surface, and the 45° mirror directs the view normal to the capsule length. The external controller drives the EPM in 2 degrees of freedom (DOF). When the EPM is rotated along vertical axis, the two IPM is attracted to follow the rotation, giving endoscope panning control. Rotating the EPM along horizontal axis would drive the IPMs to rotate along capsule length in opposite direction, achieving camera tilt similar to the magnetic actuation scheme proposed by Garbin et al. (18,20). The FOV in this design is significantly improved, as full range tilting and panning is available. The internal unit of magnetic actuated endoscope is even more lightweight (3.6 g), and compact (7 mm diameter, 40 mm length). While its length is similar to Soft MAGS endoscope, the modified actuation scheme allows tilting of camera without its length sweeping across vertical workspace, instead using rotation along body length and a mirror to redirect viewing direction normal to length. Therefore, the workspace is reduced to near the anchoring surface, significantly lowering the risk of interfering with other surgical instruments, and ideal for VATS, where space is highly constrained in transverse plane. Figure 3 shows the prototypes and testing in ex vivo set-ups. Anchoring range of current prototype is 40 mm, sufficient to cover average thoracic wall thickness; the anchoring range can be readily increased to cover overweight patients by increase of EPM size. Current prototype does not offer lens cleaning functions, therefore debris and blood stains on the mirror or lens need to be wiped off with grasper and gauze, while lens cleaning water jet and anti-fog solution can be implemented in future iteration to simplify operation. Optical prism could be used to replace 45° mirror to avoid formation of double image. For the external controller, a hands-free control strategy is under development to use foot pedals or gaze tracking as inputs to determine EPM position, and drive endoscope viewing direction. Compared to thoracoscope, this would allow surgeon to operate without addition personnel to steer the endoscope.
Conclusions
We have developed three endoscopic devices (cardioscope, Soft MAGS endoscope, and magnetic actuated endoscope) to address the need for more viewing directions, and minimize fencing and interference with other surgical instruments. Cardioscope offer a flexible articulated tip with adjustable bending length, providing wide FOV (capable of retroflexion) and changing workspace to adapt different space constraints. Soft MAGS endoscope reduces incision port occupancy all together, and made MAGS technology more feasible for surgical application by forming lightweight, compact internal unit, that is easy to produce and sterilize. The soft body can passively adapt to surroundings and is safer for patient, but the wireless camera is limited by low frame rate and short battery life, also endoscope tilting creates vertical sweeping of the device into the workspace which is not ideal for VATS. The magnetic actuated endoscope further improve the MAGS endoscope concept, with a lighter (3.6 g) and more compact (7 mm diameter) design, and only thin wire to minimize surgical port occupancy. The design has full range tilting and panning, improving FOV, and has planar workspace where camera tilt is realized with rotation along capsule length which is ideal for thoracic application considering space restrictions. While some improvements in image quality and lens cleaning strategies are needed before these devices are ready for prime time in the operating theatre, we expect these innovations to enhance surgeons’ and patients’ experience in VATS, especially in uniportal VATS of the future.
Acknowledgements
Funding: This work is supported by the Chow Yuk Ho Technology Centre for Innovation Medicine (project No. TIMSG 15/16-2), CUHK Direct grant for research (2015.2.011) and Hong Kong Innovative Technology Fund (project No. ITS/126/16).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ng CS, Lau KK, Gonzalez-Rivas D, et al. Evolution in Surgical Approach & Techniques for Lung Cancer. Thorax 2013;68:681. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Single Port Video-Assisted Thoracic Sur-gery: Advancing Scope Technology. Eur J Cardiothorac Surg 2015;47:751. [Crossref] [PubMed]
- Swing Prism Borescope. Olympus Corporation. (cited on 1st, Nov. 2017). Available online: https://www.olympus-ims.com/en/swing-prism-borescopes
- Endoeye. Olympus Corporation. (cited on 1st, Nov. 2017). Available online: https://www.olympus.se/medical/en/medical_systems/products_services/product_details/product_details_17285.jsp
- Li Z, Oo MZ, Nalam V, et al. Design of a novel flexible endoscope—cardioscope. J Mech Robot 2016;8:051014. [Crossref]
- Li Z, Ng CS. Future of uniportal video-assisted thoracoscopic surgery—emerging tech-nology. Ann Cardiothorac Surg 2016;5:127-32. [Crossref] [PubMed]
- Zhao ZR, Li Z, Situ DR, et al. Recent clinical innovations in thoracic surgery in Hong Kong. J Thorac Dis 2016;8:S618-26. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing Chest Wall Trauma in Single Port Video-Assisted Thoracic Surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Park S, Bergs RA, Eberhart R, et al. Trocar-less instru-mentation for laparoscopy: magnetic positioning of intra-abdominal camera and retractor. Ann Surg 2007;245:379-84. [Crossref] [PubMed]
- Cadeddu J, Fernandez R, Desai M, et al. Novel magnetically guided intra-abdominal camera to facilitate laparoendoscopic single-site surgery: initial human experience. Surg Endosc 2009;23:1894-9. [Crossref] [PubMed]
- Raman JD, Bergs RA, Fernandez R, et al. Complete transvaginal NOTES nephrectomy using magnetically anchored instrumentation. J Endourol 2009;23:367-71. [Crossref] [PubMed]
- Best SL, Bergs R, Scott DJ, et al. Solo surgeon lapa-ro-endoscopic single site nephrectomy facilitated by new generation magnetically anchored and guided systems camera. J Endourol 2012;26:214-8. [Crossref] [PubMed]
- Yin G, Han WK, Faddegon S, et al. Laparoendoscopic single site (LESS) in vivo suturing using a magnetic anchoring and guidance system (MAGS) camera in a porcine model: impact on ergonomics and workload. Urology 2013;81:80-4. [Crossref] [PubMed]
- Terry BS, Mills ZC, Schoen JA, et al. Single-port-access surgery with a novel magnet camera system. IEEE Trans Biomed Eng 2012;59:1187-93. [Crossref] [PubMed]
- Simi M, Pickens R, Menciassi A, et al. Fine tilt tuning of a laparo-scopic camera by local magnetic actuation: two-port nephrectomy experience on human cadavers. Surg Innov 2013;20:385-94. [Crossref] [PubMed]
- Tortora G, Dario P, Menciassi A. Array of Robots Augmenting the Kinematics of Endo-cavitary Surgery. IEEE/ASME Transactions on Mechatronics 2014;19:1821-9. [Crossref]
- Cheng T, Ng CS, Chiu P, et al. Design and Prototyping of a Soft Magnetic Anchored and Guidance Endoscope System. In Proceedings of 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). 2017: Vancouver, BC, Canada. New York: IEEE, 2017:2903-8.
- Garbin N, Di Natali C, Buzzi J, et al. Laparoscopic tissue retractor based on local magnetic actuation. J Med Devices 2015;9:011005. [Crossref]
- Liu X, Mancini GJ, Tan J. Design and analysis of a magnetic actuated capsule camera robot for single incision laparoscopic surgery. In Proceedings of 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). 2015: Hamburg, Germany. New York: IEEE, 2015:229-35.
- Garbin N, Slawinski PR, Aiello G, et al. Laparoscopic Camera Based on an Orthogonal Magnet Arrangement. IEEE Robotics and Automation Letters 2016;1:924-9. [Crossref]
Cite this article as: Cheng T, Ng CS, Li Z. Innovations in surgical scopes—wireless steerable endoscopes and magnetic cameras. Shanghai Chest 2017;1:64.


